- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2015
- Electronic structure, magnetism and dynamics
- Formation of neptunium dioxide nanocrystals under the aqueous conditions relevant to deep geological disposal
Formation of neptunium dioxide nanocrystals under the aqueous conditions relevant to deep geological disposal
The present study investigates the potential formation of colloidal species of tetravalent neptunium (Np(IV)) under the aqueous conditions relevant to the geological disposal of radioactive wastes. Np(IV) possesses an intrinsic nature to form nanosized crystalline NpO2 particles, which could potentially facilitate the transport of Np nuclides from nuclear waste repositories.
The management of radioactive wastes is still a significant challenge facing the nuclear power industry. One plausible option for the management of high-level radioactive wastes is deep geological disposal, in which the wastes are buried deep underground. Due to its long-lived radiotoxicity, a major isotope of neptunium, 237Np (T1/2 = 2.14 × 106 y), is considered to be one of the problematic radionuclides in high-level radioactive wastes in terms of the long-term safety assessment of the waste repositories. For this reason, the chemical behaviour of Np has been investigated extensively under geochemically relevant conditions [1]. One possible pathway that would potentially release radionuclides from the waste repositories into the surrounding environment is the formation of mobile colloids. As a matter of fact, such mobile colloids have been reported for plutonium (Pu), the formation of which eventually facilitates the migration of Pu for several kilometres in groundwater [2].
 |
|
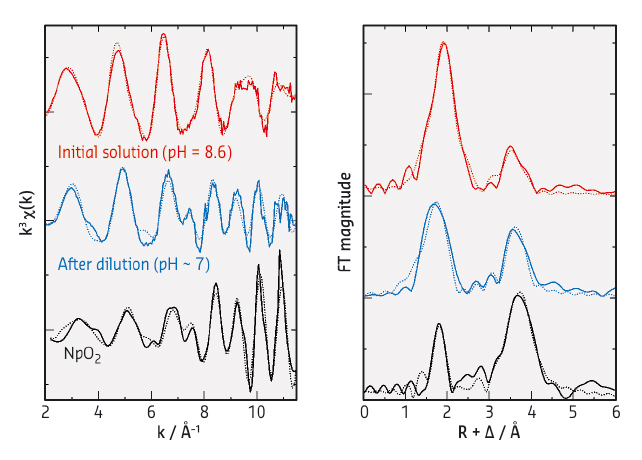
Fig. 67: (left) k3-weighted Np LIII-edge EXAFS spectra for Np(IV) in 1 M NaHCO3 at pH = 8.6 (red), after the dilution of the initial Np(IV) solution with deionised water (blue) and reference NpO2 powder (black), and (right) their corresponding Fourier transforms (FTs). Solid lines, experimental data; dotted lines, theoretical fit. The magnitude of the data for NpO2 is reduced by one forth relative to the y axes for clear comparison of the spectra. |
To characterise the Np(IV) species formed in different aqueous conditions, we performed X-ray absorption spectroscopy (XAS) at BM20 (ROBL CRG). Shown in Figure 67 are the Np LIII-edge extended X-ray absorption fine structure (EXAFS) spectra for the Np(IV) species in aqueous carbonate solutions at different pH (left, k3-weighted) and their corresponding Fourier transforms (FTs, right). The initial Np(IV) sample at pH = 8.6 (red data in Figure 67) showed a longer frequency EXAFS oscillation which produced two significant peaks in the FT. This data can be interpreted as a soluble pentacarbonate Np(IV) complex, [NpIV(CO3)5]6–. When the initial Np(IV) sample was diluted with deionised water, which also involved a slight decrease in pH from 8.6 to ~7, the resultant solution exhibited a complicated EXAFS pattern with a shorter frequency in the higher k-range (blue data in Figure 67). The observed EXAFS oscillation feature is comparable with that for NpO2 (black data in Figure 67), indicating that the Np(IV) species in the diluted solution is composed of NpO2-like structure. Additional UV-visible and light scattering measurements on this diluted Np(IV) solution revealed the formation of colloids. Hence, the Np(IV) species formed in the diluted solution is actually the colloidal particles with a NpO2-like framework.
 |
|
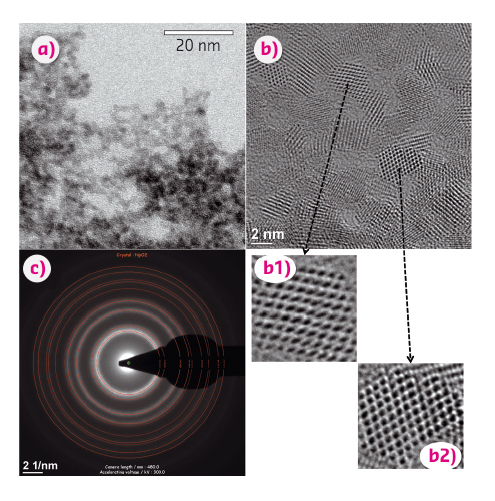
Fig. 68: (a) Bright field TEM micrograph of the dried precipitate obtained from the diluted Np(IV) solution, (b) their HR-TEM images and (c) selected area electron diffraction (SAED) patterns. Red rings in (c) are the simulated diffraction patterns based on fluorite-type NpO2 structure. |
To further characterise the colloidal Np(IV) species, high-resolution transmission electron microscopy (HR-TEM) was performed on the precipitate obtained from the diluted Np(IV) solution. Figure 68 shows typical TEM micrographs acquired from the precipitate, clearly demonstrating the agglomeration of particles with 2-5 nm in diameter (a) as well as some clear lattice images (b) which are consistent with the fluorite-type NpO2 structure (Fm3m) (c). These results, together with the EXAFS results, suggest that the dilution of the initial Np(IV) solution induces the dissociation of soluble Np(IV) species ([NpIV(CO3)5]6–), which eventually results in the formation of nano-sized NpO2 crystalline particles. This pathway of nanocrystalline NpO2 formation could be a possible scenario for the potential transport of Np nuclides from waste repositories (near field with alkaline conditions) to the surrounding environment (far field with neutral conditions) involving diffusion processes (dilution). These findings could be of particular importance in terms of the long-term prediction of radionuclide transport in geological environments, as nanosized NpO2 particles are expected to be stable for a long period of time and would potentially facilitate long-distance transport of Np nuclides from the repositories.
Principal publication and authors
R. Husar (a), R. Hübner (b), C. Hennig (a,c), P. M. Martin (d), M. Chollet (d), S. Weiss (a), T. Stumpf (a), H. Zänker (a) and A. Ikeda-Ohno (a), Chem. Comm. 51, 1301-1304 (2015); doi: 10.1039/c4cc08103j.
(a) Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Institute of Resource Ecology, Dresden (Germany)
(b) Helmholtz-Zentrum Dresden-Rossendorf (HZDR), Institute of Ion Beam Physics and Materials Research, Dresden (Germany)
(c) The Rossendorf Beamline, ESRF
(d) Commissariat à l’énergie atomique et aux énergies alternatives (CEA), Cadarache (France)
References
[1] Chemical Thermodynamics of Neptunium and Plutonium, R.J. Lemire et al., (Eds.), Elsevier Science, Amsterdam, The Netherland (2001, updated in 2003).
[2] A.B. Kersting, et al., Nature 397, 56-59 (1999).



