- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2014
- Structure of materials
- Generation of surface steps on Pt(9 7 7) induced by the catalytic oxidation of CO
Generation of surface steps on Pt(9 7 7) induced by the catalytic oxidation of CO
Atoms at surfaces have fewer neighbours than atoms in the bulk (typically 9 vs 12) and consequently they have “unsaturated” chemical bonds. Atoms at surface steps (see Figure 125) have even more “dangling” bonds (seven neighbours only) and due to this, they might be key actors in surface chemical reactions in catalysis.
This simple idea was proposed in 1925 and since that time it has been investigated extensively. Atoms at steps have been found to dissociate molecules such as N2, CO or molecules with C-C bonds; in most cases this has been determined using ultra-high vacuum techniques. However, chemical reactions are more complicated than the adsorption and dissociation process and many studies have set out to question the simple picture of “more step atoms implies more reactivity”. This was the case for several reports dealing with CO oxidation to CO2.
 |
|
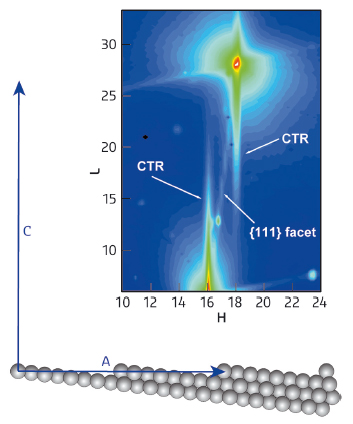
Fig. 125: a) Side view of the 977 surface showing step and terrace atoms. b) A portion of reciprocal space diffraction pattern. The diffuse CTR lines arise from the monoatomic steps and the {111} diffuse inclined line from (111) microfacets. |
We investigated the oxidation of CO at beamline ID03 using a single crystal surface of platinum as the catalyst. This crystal has a periodical array of steps (see Figure 125), which generates specific diffraction features.
Figure 125b shows a portion of the diffracted intensity. The diffuse lines at H = 16 and 18 designated as CTR (crystal truncation rod) arise from the step array, whereas the intense peaks (red colour) arise from the diffraction of bulk atoms, of no interest here. The diffuse lines marked {111} indicate that the crystal surface also has regions with (111) symmetry, therefore not all areas are stepped as depicted in the figure. Regions with (111) symmetry occur because the 977 surface is not very stable and in some places the steps bunch together generating (111) microfacets.
By measuring the intensities of the CTR and of the {111} diffuse lines, we were able to monitor the evolution of the concentration of steps at the surface during the reaction.
We used a specially designed reactor [1] that allows the diffracted intensities and the reaction product to be measured simultaneously while the reaction 2CO + O2 = 2CO2 was occurring. The proportion of the reactants was measured with the experimental variable R = PCO / PO2, the ratio of the partial pressures. R = 2 corresponds to the stoichiometric proportion and R < 2, R > 2 to oxygen rich or oxygen deficient proportions. The total pressure PCO + PCO2 = 200 mbar and the reaction temperature was around 490°C. The amount of CO2 produced was monitored with a gas spectrometer.
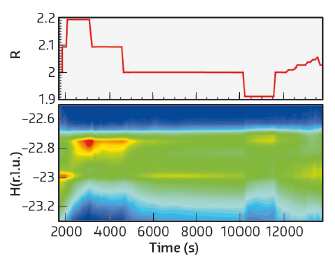
We found that, at a given temperature, the production of CO2 was maximum for R = 2 and that the maximum reactivity was associated with a maximum density of steps. Values of R other than 2 resulted in lower CO2 production and fewer steps in the Pt surface. Some of these observations are presented in Figure 126.
 |
|
Fig. 126: Temporal variation of the proportion of the reactants (upper panel) and the corresponding changes in reciprocal space (lower panel). The intensity at H = –23 arises from steps and that at ~ –22.7 from (111) microfacets. At the start of the experiment (R = 2, stoichiometric mixture), the intensity from the monoatomic steps is pronounced (red indicates high intensity) and it fades out when the gas mixture is made CO rich, while the intensity from the facets that was initially low (green) clearly develops. When the gas mixture is set back to the stoichiometric proportion (near t = 5000 s), the intensity from the steps increases again (yellow segment), while that of the facets strongly decreases. Subsequent change to an oxygen rich mixture (t ≅ 10,000 s) results again in a decrease in the intensity from the steps in favour of the facets. Finally, the intensity from the steps reappears when the gas mixture is returned to the stoichiometric value. |
These results illustrate that the morphology of the catalyst changes with the reaction conditions. When the reactivity is high, the number of steps is also high, whereas when the reactivity is low, the number of steps decreases and the proportion of (111) microfacets increases. The catalyst is not a passive spectator of the reaction, rather the contrary, it transforms itself to optimise the reaction rate by increasing its proportion of step atoms. Thus, the atoms at the steps do appear to be a key ingredient for a high reaction rate.
Principal publication and authors
O. Balmes (a), G. Prevot (b), X. Torrelles (c), E. Lundgren (d) and S. Ferrer (c,e), Journal of Catalysis 309, 33–37 (2014).
(a) ESRF
(b) Institut des NanoSciences de Paris, UMR CNRS 7588, Université Pierre et Marie Curie-Paris 6 (France)
(c) Institut de Ciència de Materials, CSIC, Bellaterra (Spain)
(d) Department of Synchrotron Radiation Research, Institute of Physics, Lund University (Sweden)
(e) ALBA Light Source, Cerdanyola del Valles (Spain)
References
[1] R. van Rijn et al., Rev, Sci. Instrum. 81, 014101 (2010).



