- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Structural biology
- Structural analysis reveals the LxxLL motif hijacking strategy developed by papillomavirus E6 oncoproteins
Structural analysis reveals the LxxLL motif hijacking strategy developed by papillomavirus E6 oncoproteins
High-risk mucosal human papillomaviruses (hrm-HPVs) provoke cervical cancers [1] by means of two small multifunctional oncoproteins named E6 and E7. hrm-HPV E6 recruits the ubiquitin ligase E6AP and tumor suppressor p53, leading to ubiquitin–mediated degradation of p53 [2]. E6 captures E6AP and many other cellular proteins through acidic leucine-rich motifs containing the LxxLL consensus sequence [2]. Despite its small size, 150 residues, E6 is prone to aggregation, which has long precluded its structural analysis.
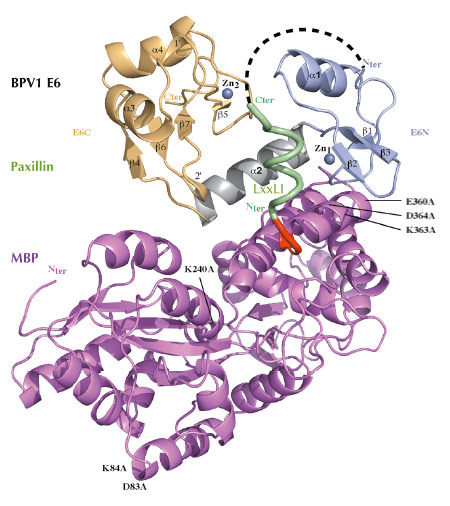
We designed strategies to solubilise two E6 proteins from HPV16 (the most tumorigenic HPV type) and BPV1 (an oncogenic bovine papillomavirus). We used the target LxxLL peptide as a solubilising ligand, fused our constructs to the highly soluble and crystallisation prone Maltose-Binding Protein (MBP), and also mutated, on the surface of HPV16 E6, four cysteine and one phenylalanine residues, which we had previously found to promote E6 self-association [3]. This allowed us to crystallise BPV1 E6 and HPV16 E6, respectively bound to LxxLL peptides from the focal adhesion protein paxillin and the ubiquitin ligase E6AP. The structures (Figures 50 and 51) were solved at resolutions of 2.3 Å and 2.6 Å, respectively, by molecular replacement using the known structure of MBP as a template.
 |
|
Fig. 50: Crystal structure of a triple fusion protein comprising a crystallisation-prone mutant of MBP, a LxxLL motif from paxillin, and wild-type bovine (BPV1) E6 oncoprotein. Crystallisation-promoting MBP mutations are indicated. Red: triple alanine linker. Green: helical LxxLL motif, corresponding to the 10 N-terminal residues of focal adhesion protein paxillin. Light blue: N-terminal zinc binding domain. Grey: linker helix. Gold: C-terminal zinc binding domain. |
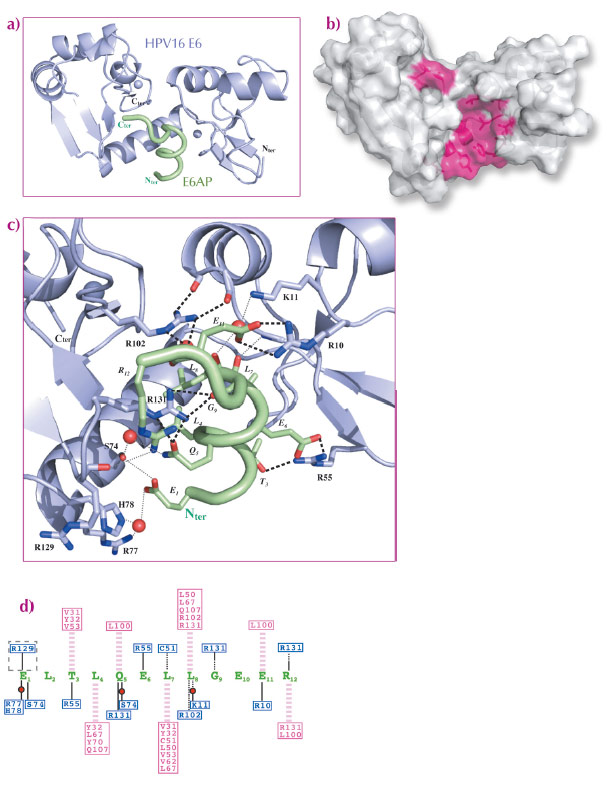
The two structures are very similar (Figure 50, Figure 51a). E6 comprises two zinc-binding domains joined by a linker helix (Figure 50, Figure 51a). The LxxLL motif adopts a a-helical conformation and docks within a hydrophobic cavity (Figure 51b) formed by the two zinc-binding domains and the linker helix. Surrounding the cavity, several polar or charged residues are involved in maintaining the architecture of the complex (Figure 51c). Mutagenesis of a number of polar or charged residues lining the pocket, including several key arginines, disrupted binding of both E6 proteins to the LxxLL motif as well as their oncogenic activities (cellular transformation for BPV1 E6 and p53 degradation for HPV16 E6). Mutagenic data combined with computational analysis of both complexes allowed us to distinguish two distinct roles for the peptide-contacting residues of E6. i) Conserved pocket residues accomodate the invariable leucine side chains and establish sequence-independent contact to the peptide’s main chain. ii) Variable “reader” residues discriminate variations of the LxxLL motif through sequence-dependent contacts to variable side-chains of the bound peptide. Such combinations of discriminative and tolerant reading mechanisms clearly must allow different E6 proteins to capture different panels of host proteins bearing variations of the LxxLL motif.
 |
|
Fig. 51: Crystal structure of a soluble mutant of HPV16 E6 in complex with LxxLL motif of E6AP. a) Blue: E6; green: LxxLL peptide. MBP is not represented. b) The LxxLL-binding hydrophobic pocket. c) Networks of polar interactions between HPV16 E6 and LxxLL motif of E6AP. d) A summary of all contacts between HPV16 E6 and the LxxLL motif of E6AP. Pink dashed lines: hydrophobic contacts; black lines: polar contacts mediated by side chain (continuous lines) or main chain (dotted lines); pink/blue boxed residues: E6 hydrophobic/polar contributors. |
The hijacking of cellular motifs is a well-known viral strategy [4]. Most frequently, viral proteins evolve small sequence fragments, which mimic cellular linear motifs, thereby interfering with their functions. In contrast, PV E6 has evolved an entire pocket, composed of two folded domains, which captures cellular LxxLL motifs to perform oncogenic functions. The LxxLL-binding pocket of E6, revealed in atomic detail by this work, therefore represents a promising target for therapeutic drugs against HPV-induced cancers.
Principal publication and authors
K. Zanier (a), S. Charbonnier (a), A.O.M.O. Sidi (a), A.G. McEwen (b), M.G. Ferrario (b), P. Poussin (b), V. Cura (b), N. Brimer (c), K.O. Babah (a), T. Ansari (c), I. Muller (a), R.H. Stote (b), J. Cavarelli (b), S. Vande Pol (c) and G. Travé (a). Science 339, 694-8 (2013).
(a) UMR 7242, Biotechnologie et Signalisation Cellulaire, École Supérieure de Biotechnologie de Strasbourg, Illkirch (France)
(b) Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) / INSERM U964 / CNRS UMR 7104/ Université de Strasbourg, Illkirch (France)
(c) Department of Pathology, University of Virginia, Charlottesville (USA)
References
[1] H. zur Hausen. Semin Cancer Biol 9, 405 (1999).
[2] S.B. Vande Pol and A.J. Klingelhutz. Virology 445, 115-137 (2013).
[3] K. Zanier et al.. Structure 20, 604 (2012).
[4] N.E. Davey, G. Trave and T.J. Gibson, Trends Biochem Sci 36, 159 (2011).



