- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Dynamics and extreme conditions
- Reactivity of Xenon with ice under planetary conditions
Reactivity of Xenon with ice under planetary conditions
Noble gases are characterised by their high chemical stability. It is both interesting and challenging to force them into bonding with other atoms because of their lack of reactivity. Furthermore, the chemical bonds they form are highly energetic. This has fuelled the search for high-energy noble gas compounds, and, since Neil Bartlett made the first xenon compound (Xe+[PtF6]-) in 1962, over 100 noble gas compounds have been synthesised, the vast majority involving xenon. One of the most recently discovered compounds is the HXeOXeH molecule [1], in which two xenon atoms are inserted into a water molecule. It was synthesised by the reaction between water and xenon, driven by UV light at low temperatures (below 70 K). Instead of using very low temperature, another way to make unstable molecules exist for longer periods is to create them under very high pressure. For xenon, there is a large deficiency in the atmospheres of Earth, Mars [2] and Jupiter, the only planets for which noble gas content has been measured. This is the so-called ‘missing Xe paradox’, which suggests that xenon is retained inside the planets, i.e. under high pressure conditions.
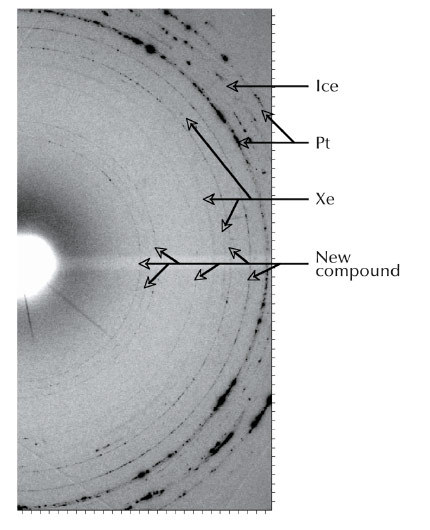 |
|
Fig. 33: 2-D diffraction pattern obtained at 58 GPa and 1500 K, new rings are visible. |
Using laser-heated diamond anvil cells at beamline ID27, we have discovered that xenon reacts with water ice at pressures above 50 GPa and temperatures of 1500 K (Figure 33) – conditions found in the interiors of Uranus and Neptune, and in the terrestrial lower mantle. In situ X-ray diffraction revealed the presence of a hexagonal lattice with four xenon atoms per unit cell and several possible distributions of oxygen atoms. We combined the diffraction data with ab initio calculations in order to solve the crystallographic structure of the compound. Figure 34 shows the Xe2O6H6 unit on which the compound is based.
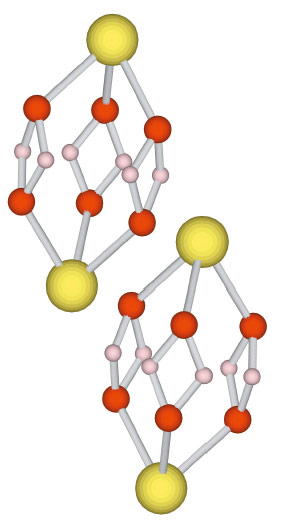 |
|
Fig. 34: The new compound contains two Xe2O6H6 units per unit cell. |
This discovery follows an earlier report of the stability of Xe-doped quartz (SiO2) at the conditions prevalent deep under the continents on Earth [3], which was confirmed by the later synthesis of pure XeO2 [4]. This questions in turn the use of xenon isotopes to determine events in the formation of the Earth. Noble gas abundances are widely used by geochemists to assess the order and timing of major terrestrial processes, including atmospheric formation. However, their basic assumption is that noble gases remain inert under all conditions. The present findings show that the chemistry of Xe is much more complex than previously thought.
Principal publication and authors
C. Sanloup (a,b), S.A. Bonev (c,d), M. Hochlaf (e), and H.E. Maynard-Casely (f), Phys. Rev. Lett. 110, 265501 (2013).
(a) Institut des Sciences de la Terre Paris, UPMC Univ Paris 06, Paris (France)
(b) School of Physics, University of Edinburgh, Edinburgh (UK)
(c) Lawrence Livermore National Laboratory, Livermore (USA)
(d) Department of Physics, Dalhousie University, Halifax (Canada)
(e) Laboratoire Modélisation et Simulation Multiéchelle, Université Paris-Est, Marne-la-Vallée (France)
(f) Bragg Institute, Australian Nuclear Science and Technology Organisation, Lucas Heights, (Australia)
References
[1] L. Khriachtchev et al., J. Am. Chem. Soc. 130, 6114 (2008).
[2] E. Anders and T. Owen, Science 198, 453 (1977).
[3] C. Sanloup et al., Science 310, 1174 (2005).
[4] D.S. Brock and G.J. Schrobilgen, J. Am. Chem. Soc. 133, 6265 (2011).



