- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- X-ray imaging
- Particle redistribution and defect development during the freezing of colloidal suspensions
Particle redistribution and defect development during the freezing of colloidal suspensions
The solidification of colloidal suspensions is commonly encountered in our everyday life through a variety of natural processes such as the freezing of soil and the growth of sea ice. It is also seen in engineering applications including food engineering, cryobiology, filtration and water purification. In materials science, the freezing of colloidal suspensions is finding applications in the processing of porous materials, usually referred to as ice templating or freeze-casting. This simple process, where a colloidal suspension is simply frozen under controlled conditions and then sublimated before sintering, provides materials with a unique porous architecture, where the porosity is a replica of that of ice crystals.
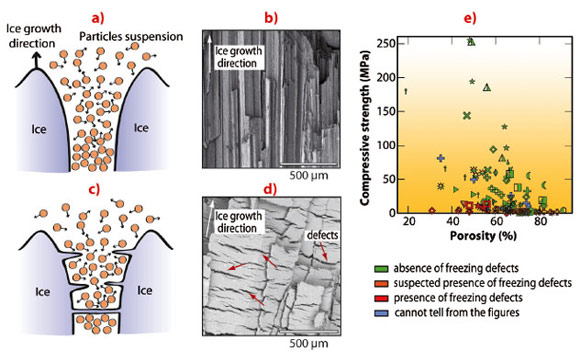
A wide range of compressive strength values is reported in the literature for ice-templated materials (Figure 62e). Microstructural observations revealed that many of the weakest samples in the literature had structural defects oriented perpendicular to the ice growth direction (Figure 62d). High-strength samples (Figure 62b) are systematically free of such defects. The absence of such defects is clearly a necessary but insufficient condition to obtain high compressive strength.
Our current understanding of the solidification of colloidal suspensions is derived primarily from investigation of the behaviour of single particles relative to a moving interface. Many geological, biological and industrial systems involve concentrated particle systems. In colloidal systems, particle–particle electrostatic interactions can strongly determine the behaviour of the system (Figure 62a). Through systematic in situ X-ray imaging, we investigated the roles of the suspension composition and the particle–particle electrostatic interactions on defect formation during the freezing of colloidal suspensions. We performed in situ observations of crystal growth and particle redistribution during freezing by X-ray radiography and tomography at beamline ID19. We show that particle–particle interaction can have a dramatic influence on the mechanisms controlling the formation of the structure.
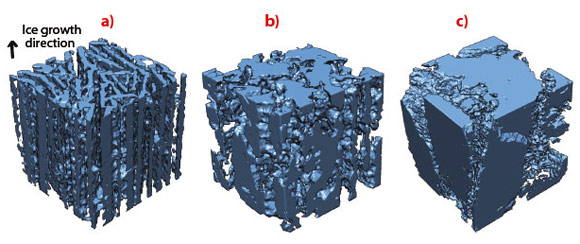
In situ radiography of the advancing freezing front shows a behaviour that is highly dependent on the components added in the initial suspension. 3-D tomographic reconstructions of frozen samples show a range of microstructure, from disoriented ice crystals (Figure 63c) to aligned, regular ice crystals (Figure 63a) corresponding to stable growth (Figure 62a). Defects are observed perpendicular to the freezing direction (Figure 62d, Figure 63b), forming pores that traverse the dense ceramic walls (Figure 62d), significantly affecting the integrity of the structure. The development of defects perpendicular to the main ice growth direction must be avoided to obtain a strong structure. Materials obtained under conditions promoting the development of such defects in the microstructure are practically useless. The perpendicular defects created here in ice-templated materials strongly resembles the ice lenses observed in geophysics. Ice lenses are ice crystals observed in frozen soils or during the directional solidification of colloidal suspension, growing perpendicular to the temperature gradient direction (Figure 62c). Since the porosity found here is a replica of the ice crystal network obtained after freezing, ice lenses will result in the presence of crack-like pores perpendicular to the main ice growth direction.
The formation of defects was correlated with in situ observations revealing the apparition of flocculated and depleted zones in the concentrated suspension during freezing. Our results show that flocculation can be a viable mechanism to facilitate ice lens nucleation and growth in such a system, and are a first step towards incorporating particle–particle electrostatic interactions into our understanding and modelling of the freezing of colloids.
Principal publication and authors
A. Lasalle (a), S. Deville (a), C. Guizard (a), E. Maire (b) and J. Adrien (b), Acta Materialia 60, 4594–603 (2012).
(a) Laboratoire de Synthèse et Fonctionnalisation des Céramiques, CNRS, Cavaillon (France) (b) MATEIS, Université de Lyon, Villeurbanne (France)