- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Electronic structure and magnetism
- Size influence on Pd catalysis for pollutant abatement
Size influence on Pd catalysis for pollutant abatement
Pd-based three-way catalysts are a paradigmatic example of a technology for controlling pollutant emissions, specifically the removal of noxious NOx emissions and the complete oxidation of hydrocarbons and CO to CO2 in gasoline engine powered vehicles. Catalyst operation under light-off or isothermal conditions has been exhaustively evaluated but in recent years the focus has shifted to more realistic, dynamic, cyclic conditions. Among the major issues targeted by early studies under cycling conditions, the key role of redox chemistry processes in controlling catalytic activity was clearly established. More recent studies have however revealed a significantly richer chemistry than may have been previously supposed. Particularly for CO elimination, there is intensive activity both at basic and applied levels that indicates that CO oxidation can involve a series of unexpected steps. For CO/NO cycling conditions, a role for a Pd carbide phase resulting from CO dissociation has been recently demonstrated [1]. Also, studies concerning the dynamics of the oscillatory catalytic behaviour upon CO/O2 mixtures have triggered a debate as to whether the most active phase relates to a surface Pd oxide layer or is a metallic one dominated by chemisorbed oxygen [2]. All these studies confirm that Pd chemistry in pollutant elimination processes needs to be further clarified from a basic perspective. A basic understanding of how supported noble metal size affects the catalytic performance was thus investigated here using cycling CO/(NO+O2) conditions.
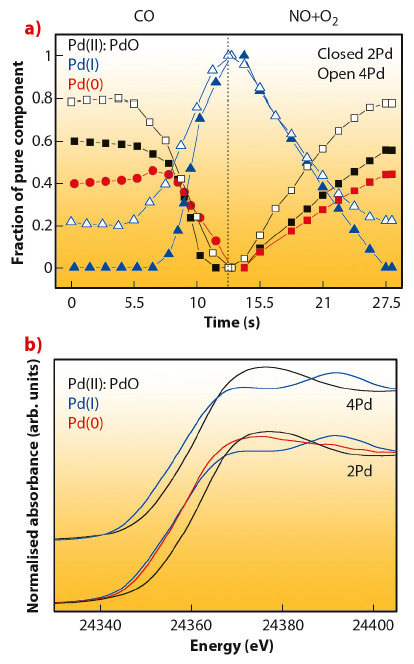
To derive a new way of seeing into the dynamic behaviour of such systems, experiments at ID24 (dispersive EXAFS) and ID15 (hard X-ray diffraction - HXRD) were carried out. Figure 89 collates XANES spectra of Pd chemical species and corresponding concentration profiles (obtained through a factorial analysis approach) during a single CO/(NO+O2) cycle at 673 K for two Pd-based three-way catalyst materials having different Pd loading (2 and 4 wt.%) on alumina and corresponding to a particle size of ca. 1.5 and 2.5-3.0 nm in the fully metallic state. Most apparent differences are related to the presence of an intermediate Pd(I)-like species in the lower Pd loading case. In particular, these species delay the appearance of Pd(0) under the CO atmosphere with respect to the higher loaded sample. Such differential behaviour is confirmed using two additional techniques, EXAFS and HXRD (see principal publication). The combined utilisation of these X-ray techniques shows that this Pd(I) phase corresponds to a surface oxide-containing layer or patch and has a distinctive electronic structure which retains a certain metallic character obviously based in the existence of Pd-Pd contacts.
 |
|
Fig. 89: XANES spectra (top) and concentration profiles (bottom) corresponding to pure chemical species present during a single CO/(NO+O2) cycling treatment at 673 K. |
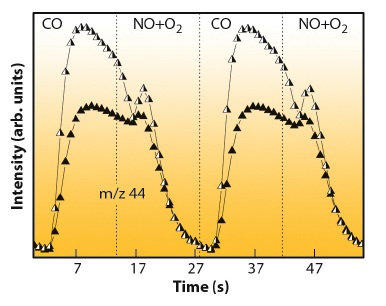
Catalytically speaking, the presence of such Pd(I) phase is rather important. Figure 90 plots the normalised (per surface active centre as measured by XRD/EXAFS) activity for CO2 production for the two Pd-based materials under study. This provides the evidence that both Pd(I) and Pd(0) species are highly active species in CO elimination but the superior performance of Pd(I) is clearly established.
 |
|
Fig. 90: Normalised mass spectrometry profile for CO2 (m/z 44) production during CO/(NO+O2) cycling treatment at 673 K. Half-closed symbols: 2Pd; closed symbols; 4Pd. |
Summarising, the multitechnique XAS/HXRD/DRIFTS/MS analysis of Pd-Al2O3 systems have revealed a series of size-dependent phenomena at Pd nanoparticles upon CO/(NO+O2) cycling conditions. The most important is that both Pd(I) and Pd(0) species can be present in Pd surface layers but this is strongly dependent on the size of the nanoparticles. Both species appear to be highly active species in CO elimination but our results suggest a higher activity for the Pd(I) species. A Pd(I) chemical nature can be thus associated to the CO elimination “active” surface Pd oxide layers, at least in nanoparticulate materials below ca. 2-3 nm in diameter. Above this size limit, we detected the exclusive presence of Pd(0) as active species for CO elimination at the surface of Pd nanoparticles. These results would point to the ability to tune and maintain Pd nanoparticles of a certain size as being crucial to the most efficacious oxidation of CO during catalyst operation.
Principal publication and authors
I. Iglesias-Juez (a), A. Kubacka (a), M. Fernández-García (a), M. Di Michiel (b) and M.A. Newton (b), J. Am. Chem. Soc. 133, 4484 (2011).
(a) Instituto de Catálisis y Petroleoquímica, CSIC, C/Marie Curie 2, Madrid (Spain)
(b) ESRF
References
[1] M.A. Newton, M. Di Michiel, A. Kubacka and M. Fernández-García, J. Am. Chem. Soc. 132, 4540 (2010); A. Kubacka, A. Martínez-Arias, M. Fernández-García, M. Di Michiel and M.A. Newton, J. Catal. 270, 275 (2010).
[2] B.L.H. Hendriksen, M.D. Ackermann, R. van Rijn, D. Stolz, I. Popa, O. Balmes, A. Resta, D. Werneille, R. Felici, S. Ferrer and J.W.M. Frenken, Nat. Chem. 2, 730 (2010); F. Gao, Y. Cai, K.K. Gath, Y. Wang, M.S. Chen, Q.L. Guo and D.W. Goodman, J. Phys. Chem. C 113, 182 (2009).



