- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- Materials Science
- Resonant Contrast Diffraction for Direct Localisation of Atoms in Mixed Occupancy Powders
Resonant Contrast Diffraction for Direct Localisation of Atoms in Mixed Occupancy Powders
Describing detailed atomic order in complex powders such as natural samples or industrial materials (minerals, cement, catalysts) remains a challenge because X-ray powder diffraction discriminates poorly between elements with close atomic numbers occupying neighbouring sites. Here, we apply resonant contrast diffraction to complex powders via new methodologies of anomalous difference patterns for multi-wavelength refinement to accurately localise atoms, and produce dispersive difference maps to directly visualise them.
Resonant scattering variations near an absorption edge are used in Diffraction Anomalous Fine Structure (DAFS) [1], and to solve the structure factor phase problem in biocrystallography. The present contribution focuses on a third application, Resonant Contrast Diffraction (RCD), using the chemical sensitivity of resonant diffraction to extract the contribution of a single element to each crystallographic site.
The specificities of RCD were demonstrated on powders in the nineties [2], but ab initio structure determination using partial Patterson density maps and “maximum entropy methods” becomes inefficient for complex powders due to reflection overlap [3]. Partial structure factor analysis on the other hand, is efficient in studying poorly crystallised or amorphous samples [4]. Here, we develop resonant scattering for highly-crystallised solids of industrial interest: bicationic X zeolites.
Our methodology, using two methodological advances and simultaneous refinement of several diffraction patterns, was validated by the determination Sr2+ and Rb+ cation distributions in SrRbX. Since Sr2+ and Rb+ have the same number of electrons (Z = 35 e.u.) and similar neutron scattering lengths (b(Sr) = 0.702 x 10-12 cm; b(Rb) = 0.709 x 10-12 cm) this is a particularly difficult case for conventional X-ray or neutron diffraction.
Diffraction experiments (on BM2 using Debye-Scherrer geometry) were performed 10 eV below the Rb and the Sr K edges and at 14.8 keV, far below both edges. An initial Fourier map calculated using the known zeolite structure (Figure 54a), gave rough and non element selective localisation of extra-framework atoms (cations, water molecules, etc). These are introduced as electron densities, with no assumption of the atom type, on sites I, I’, II, II’ and III (Figure 54b).
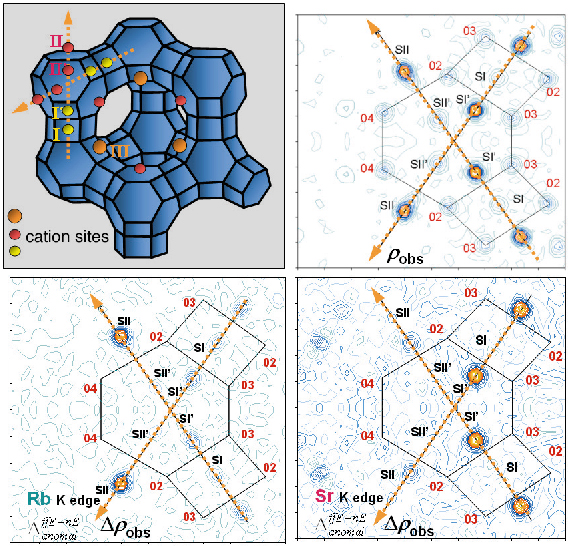 |
|
Fig. 54: The X zeolite structure: (a) the main cationic sites in the unit cell, (b) Intersection of two <111> axes defines the Fourier map section, (c) Dispersive difference map of Rb, (d) Dispersive difference map of Sr. |
Resonant diffraction gives dispersive difference map between electron densities determined at Rb and Sr edges, with amplitudes proportional to the variation of the real resonant contribution (![]() f’), allowing resonant cation localisation (Figures 54c and 54d).
f’), allowing resonant cation localisation (Figures 54c and 54d).
This selectivity can be seen directly in anomalous difference patterns calculated from data at EK-Sr – 10 eV and EK-Sr – 1300 eV (and at EK-Rb – 10 eV and EK-Rb – 400 eV), used in the refinement. Calculated and measured patterns, highly-sensitive to resonant atom location and occupancy, present close agreement after adjusting resonant atom occupancies (Figure 55).
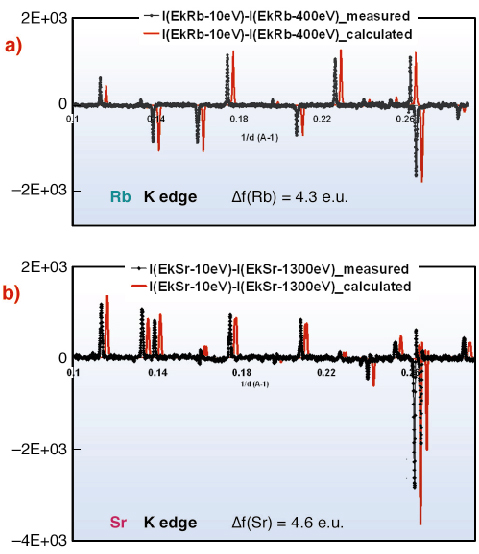 |
|
Fig. 55: Measured and calculated anomalous differential patterns (difference between diagrams obtained at different energies) at Rb- (a) and Sr- (b) K absorption edges, using the same intensity normalisation. |
Using Resonant Contrast Diffraction, cation distribution was determined in several bicationic zeolites (SrRbX, SrCaX and CaRbX). Sr2+ occupies preferentially sites I’, II and III whereas Rb+ occupies mainly site II and (if enough Rb+ is present) supercage site III. Ca2+ shows strong preference for sites I’ and III. In all solids, site II’ is mainly populated by water molecules.
In conclusion, resonant scattering enables accurate localisation of atoms in powders via dispersive difference maps in real space to locate resonant cations, and anomalous differential diffraction patterns in reciprocal space to quantify the structural model. Using this new methodology, Resonant Contrast Diffraction becomes an easily applicable key method to analyse site occupancies by chemically different cations.
References
[1] J.L. Hodeau, V. Favre-Nicolin, S. Bos, H. Renevier, E. Lorenzo, J.F. Berar, Chem. Rev. 101, 1843 (2001).
[2] D.E. Cox, A.P. Wilkinson, In: Resonant anomalous diffraction X-ray scattering. Theory and applications. G. Materlik, C.J. Sparks and K. Fischer (Eds.), 195 (1994).
[3] K. Burger, D. Cox, R. Papoular, W. Prandl, J. Appl. Crystrallogr. 31, 789 (1998).
[4] E. Matsubara, Y. Waseda, In: Resonant anomalous diffraction X-ray scattering. Theory and applications. G. Materlik, C.J. Sparks and K. Fischer (Eds.), 345 (1994).
Principal Publication and Authors
H. Palancher (a,b), J.L. Hodeau (b), C. Pichon (a), J.F. Bérar (b,c), J. Lynch (a), B. Rebours (a) and J. Rodriguez-Carvajal (d), Angew. Chem. Int. Ed., 44, 1725-1729 (2005).
(a) Institut Français du Pétrole, Vernaison (France)
(b) Lab. de cristallographie, CNRS, Grenoble (France)
(c) French CRG D2AM, ESRF
(d) Lab. Léon Brillouin, CEA/Saclay, Gif sur Yvette (France)



