- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- X-ray Imaging and Optics
- Microspectroscopy of Sulfur Helps Conservation of Henry VIII's Warship Mary Rose
Microspectroscopy of Sulfur Helps Conservation of Henry VIII's Warship Mary Rose
The Mary Rose served successfully for 35 years in Henry VIII’s navy. In 1545, when preparing for battle with a French fleet, she suddenly capsised and sank outside Portsmouth, U.K. The starboard side of the hull, salvaged in 1982, has been sprayed with an aqueous solution of polyethylene glycol (PEG) for the last 10 years, to prevent the waterlogged wood from cracking in the final drying stage of conservation (Figure 154). The report of tons of reduced sulfur compounds slowly forming acid in the almost intact hull of the 17th century Swedish warship Vasa gave rise to new concerns [1], and core samples taken from the Mary Rose’s hull revealed about 1 mass% sulfur throughout the oak timbers (~ 280 tons). When atmospheric oxygen gains access to the moist wood in the presence of catalytically-active iron ions, this starts the acid-producing oxidation of the reduced sulfur compounds.
 |
|
Fig. 154: Hull timbers of the Mary Rose being sprayed with PEG solution (photo F. Jalilehvand). |
The total sulfur and iron concentrations were determined by several methods in cores (0.4 x 15 cm) sampled from the Mary Rose hull timbers [2]. Sulfur K-edge X-ray absorption near edge structure (XANES) spectra of segments along the cores were measured at Stanford Synchrotron Radiation Laboratory. They permitted the relative amounts of sulfur in different functional groups to be evaluated by curve fitting with normalised XANES spectra of known standard compounds. Often several types of reduced sulfur species contribute to a major XANES peak at ~ 2473 eV: thiols (R-SH), disulfides (R-SS-R), elemental sulfur (S8), occasionally pyrite (FeS2) and other iron sulfides. Magazine stored timber and core surfaces show minor amounts of sulfonates (R-SO3-) and sulfate (SO42-) at 2483 eV, while hull timber under spray treatment has almost no sulfate. Sulfoxides (R(SO)R), absorbing at 2476 eV, are intermediate organosulfur oxidation products occurring in a few % in all XANES spectra.
Thin wood slices were examined using scanning X-ray absorption microspectroscopy (SXM) at ESRF beamline ID21. Raster scanning with focused X-rays at characteristic sulfur XANES resonance energies were used to create SXM images, revealing that reduced sulfur species (at 2473 eV) are concentrated in the lignin-rich middle lamella between the wood cells, and also in a distinct double layer in the lignin-reinforced walls of a vessel, which is a transport channel in oak wood. Focused micro-XANES spectra confirmed that the organosulfur consists of thiols and disulfides, and occasionally iron sulfides in separate particles (Figure 155).
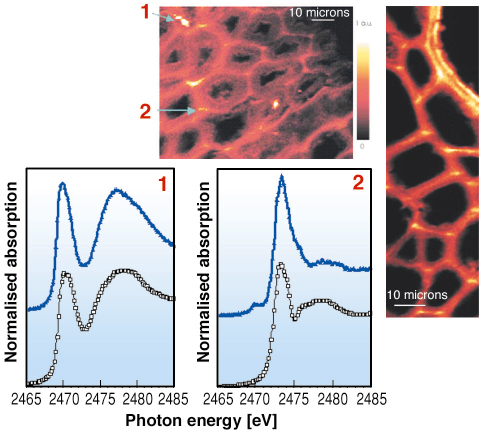 |
|
Fig. 155: Scanning X-ray microspectroscopy images at 2473 eV of reduced sulfur (brighter color |
Dissolved hydrogen sulfide (or HS-), produced by bacteria reducing sulfate ions in anoxic seawater, converts in the waterlogged wood to solid sulfur compounds in amounts depending on the state of wood degradation, the concentration of hydrogen sulphide, and of iron(II) ions. The microcrystalline iron sulfides formed in the presence of corroding iron, are known to be unstable toward oxidation in a humid environment, and are probably the primary source of acid.
For the Mary Rose the spraying washes out the acid, and the recirculated PEG solution is monitored to assess when strongly acid-forming sulfur species are exhausted. Antioxidants are being evaluated for subsequent stabilisation of the lignin-bonded organosulfur compounds and also to prevent PEG degradation. In the international “Preserve the Vasa” project, tests are being carried out to remove iron with efficient chelates [2], or in other ways retard the oxidation processes. The sulfuric acid accumulated in the Vasa’s hull timbers after the spray treatment was stopped in 1979, is estimated to be about 2 tons. Neutralising that acid with an ammonia gas treatment, followed by storage in stable and low (~ 55%) relative humidity, may be sufficient to hold back the production of new acid and possible wood degradation effects. Synchrotron-based analyses will continue to play an important role for the development of conservation methods for historical shipwrecks and marine-archaeological artefacts.
References
[1] M. Sandström, F. Jalilehvand, I. Persson, U. Gelius, P. Frank, I. Hall-Roth, Nature 415, 893-897 (2002); see also Feb. 2002 SSRL Science Highlights: http://www-ssrl.slac.stanford.edu/research/highlights_archive/vasa.html.
[2] M. Sandström, Y. Fors, F. Jalilehvand, E. Damian, U. Gelius, in Proceedings of the 9th ICOM Group on Wet Organic Archaeological Materials Conference, Copenhagen 2004, P. Hoffmann et al. (Eds.), Deutsches Schiffahrtsmuseum, Bremerhaven, 2005, pp. 181-199; see also: http://www.fos.su.se/~magnuss/.
Principal Publication and Authors
M. Sandström (a), F. Jalilehvand (b), E. Damian (a), Y. Fors (a), U. Gelius (c), M. Jones (d) and M. Salomé (e), PNAS 40, 14165-14170 (2005).
(a) Structural Chemistry, Stockholm University (Sweden)
(b) Department of Chemistry, University of Calgary (Canada)
(c) Department of Physics, Uppsala University (Sweden)
(d) The Mary Rose Trust, HM Naval Base, Portsmouth (U.K.)
(e) ESRF



