- Home
- News
- Spotlight on Science
- Revealing the dynamics...
Revealing the dynamics of active species in electrocatalysts under operando conditions
27-06-2022
X-ray absorption spectroscopy at beamline BM31 was used to investigate the dynamics of true active species in cobalt phosphide/chalcogenide-based electrocatalysts under reaction conditions. The results offer new insights that could lead to the design of higher-performing catalysts to produce green hydrogen fuel.
Share
The design of efficient electrocatalysts for industrial water splitting (i.e., separating H20 into oxygen and hydrogen) is essential to generate sustainable hydrogen fuel. However, a comprehensive understanding of the complex catalytic mechanisms under harsh reaction conditions remains a major challenge. Over the past decades, several types of electrocatalysts have been designed for large-scale and low-cost hydrogen production. Among these, transition metal chalcogenides (TMCs) and phosphides (TMPs) show remarkable catalytic performance for both the hydrogen and oxygen evolution reactions ((HER and OER respectively), thus emerging as excellent candidates for practical alkaline exchange membrane (AEM) electrolyser systems [1].
Nevertheless, TMCs and TMPs undergo structural reconstructions during the reaction process, which renders the identification of their real catalytically active phases extremely difficult. Previous studies have shown strong correlation between the local electronic structure of the materials and their catalytic activity [2]. It is likely that the main phenomenon behind the excellent activity of TMCs/TMPs catalysts for OER is structural reconstruction into metal oxides/(oxy)hydroxides [3], although it is still unknown whether these are the real active phases for OER. In addition, whether the catalysts can also undergo structural reconstructions during HER still requires in-depth investigation. Recent advances in in-situ/operando techniques have established new routes to understanding catalytic structure-activity relationships on an atomic-resolution scale for guidance in the design of high-performance electrocatalysts [4].
The catalytic mechanisms of TMC and TMP was studied using operando Raman spectroscopy and X-ray absorption spectroscopy (XAS) at beamline BM31 to track the dynamics of true active species in cobalt phosphide/chalcogenide-based electrocatalysts under operando reaction conditions and to reveal the real catalytically active intermediates during water splitting. XAS tests were carried out with two representative samples for HER and OER, namely cobalt phosphides (referred to as Co-P material) and Co-P with partial Fe substitution (referred to as Co@CoFe-P material).
Click to enlarge image.
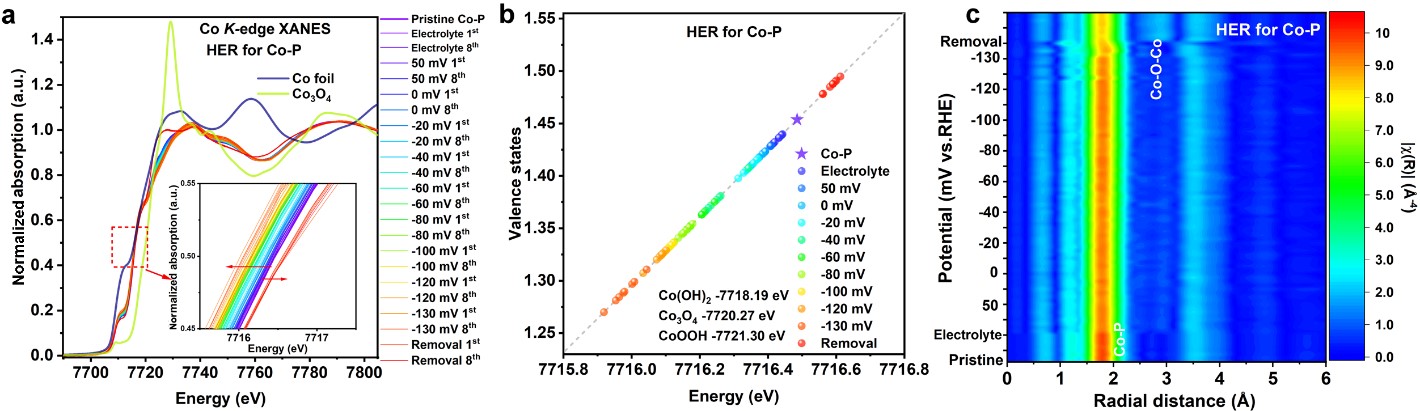
Fig. 1: Operando XAS characterisations of Co-P for HER. a) Operando Co K-edge XANES spectra of Co-P during HER. b) Calculated Co valence state vs. energy positions. c) 2D contour plots of Co K-edge FT-EXAFS spectra of Co-P during HER. Reproduced with permission from Energy Environ. Sci. 15, 727-739 (2022).
Figure 1 illustrates operando XAS characterisations of Co-P under operando HER conditions. For the HER, the rising absorption edge energy position of the operando Co K-edge XANES spectra featured a negative energy shift, indicating the formation of low-valent Co ions during the HER process (Figure 1a,b). Close inspection of the operando Co K-edge FT-EXAFS spectra (Figure 1c) revealed that the backscattering of the first coordination shells arising from Co-P pairs underwent peak profile changes during the HER. Further, after removal of the applied potential, a prominent backscattering peak in the second coordination shell arising from Co-Co pairs (present in Co-O-Co moieties) was observed in the operando FT-EXAFS spectra. This suggests that an oxidised form of Co-P featuring P-Co-O structural moieties comprising low-valent Co0/Co+ species performed as active HER catalyst.
In sharp contrast, operando XAS characterisations of Co-P during OER pointed to a series of dynamic structural transformations: Co-P → Co-P6-xOx → Co-P6-xOx@Co(OH)2@CoOOH → Co-P6-xOx@Co(OH)2 (transient)@CoOOH → CoOOH → CoO2, where the in-situ reconstructed CoO2 species with high-valent Co4+ centres served as the true OER active phases.
Click to enlarge image.
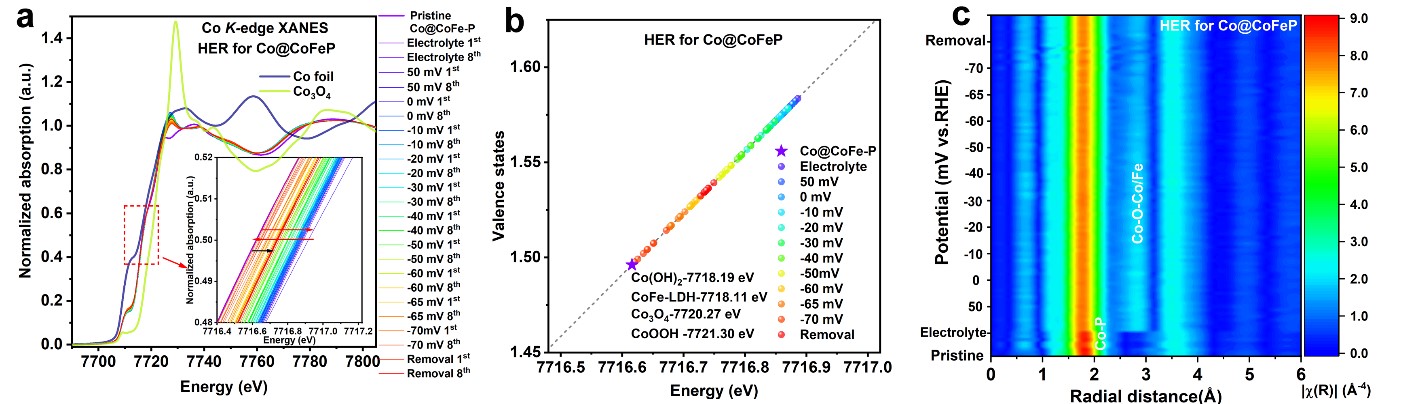
Fig. 2: Operando XAS characterisations of Co@CoFe-P for HER. a) Operando Co K-edge XANES spectra of Co@CoFe-P during HER. b) Calculated Co valence state vs. energy positions. c) 2D contour plots of Co K-edge FT-EXAFS spectra of Co@CoFe-P during HER. Reproduced with permission from Energy Environ. Sci. 15, 727-739 (2022).
Additionally, operando XAS experiments with Co@CoFe-P for HER and OER were carried out to reveal the influence of secondary metal substitution on the electrochemical properties. This is a promising approach to improve both the catalytic activity and stability of catalysts with a single metal type. Similar to the operando XANES characterisation of Co-P during HER (Figure 1a,b), the operando Co K-edge XANES spectra of Co@CoFe-P (Figure 2a,b) also demonstrates the reduction of Co centres to a low-valence state. Analysis of operando Co-K edge FT-EXAFS spectra (Figure 2c) suggested that the in-situ reconstructed P-Co-O-Fe-P structural moieties played a key role during the operando HER tests.
In conclusion, synchrotron X-ray spectroscopy can be used to track the dynamics of the local coordination environments of metal centres and also to capture the key reactive intermediates during the electrolysis process. Such investigations will hold the key to deciphering the intrinsic catalytic mechanisms of important heterogeneous catalysts as a step towards improving technical electrolysers and other areas of green hydrogen production.
Principal publication and authors
Dynamics and control of active sites in hierarchically nanostructured cobalt phosphide/chalcogenide-based electrocatalysts for water splitting, Y. Zhao (a), N. Dongfang (a), C.A. Triana (a), C. Huang (a), R. Erni (b), W. Wan (a), J. Li (a), D. Stoian (c), L. Pan (d), P. Zhang (e), J. Lan (a), M. Iannuzzi (a), G.R. Patzke (a), Energy Environ. Sci. 15, 727-739 (2022); http://www.doi.org/10.1039/D1EE02249K
(a) Department of Chemistry, University of Zurich, Zurich (Switzerland)
(b) Electron Microscopy Center, Empa, Swiss Federal Laboratories for Materials Science and Technology, Dübendorf (Switzerland)
(c) Swiss-Norwegian Beamlines at the ESRF, Grenoble (France)
(d) Key Laboratory of Advanced Metallic Materials of Jiangsu Province, School of Materials Science and Engineering, Southeast University, Nanjing (China)
(e) School of Electrical and Information Engineering and Key Laboratory of Advanced Ceramics and Machining Technology of Ministry of Education, Tianjin University, Tianjin (China)
References
[1] D. Li et al., Nat. Energy 5, 378-385 (2020).
[2] Z.W. She et al., Science 355(6321), eaad4998 (2017).
[3] H. Ding et al., Chem. Rev. 121(21), 13174-13212 (2021).
[4] J. Timoshenko et al., Chem. Rev. 121(2), 882-961 (2021).
|
About the beamline: BM31 The Swiss-Norwegian beamline BM31 is specialised in studying materials chemistry by in-situ or operando multi-probe synchrotron techniques. The main X-ray methods offered are X-ray absorption spectroscopy and powder diffraction, soon total scattering will be added. The beamline is designed to combine these three techniques in one experiment while studying processes such as heterogeneous and electro catalysis, CO2 capture and activation and energy storage. The unique combination of these three techniques enables users to probe a material’s structure, often complex and dynamic, at very different length and time scales while simultaneously determining the material’s performance. Such experiments are key to obtain fundamental structure-performance relationships in novel, technologically relevant materials under operating conditions. |