- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- X-ray imaging
- Phase contrast imaging of lipid membranes in solution
Phase contrast imaging of lipid membranes in solution
Biomolecules are well known to form large scale supra-molecular aggregates by self-assembly, forming functional units made of non-covalent bonds. Functional properties in biomolecular assemblies depend on the molecular to supra-molecular structure. An excellent example of supra-molecular assembly is the formation of lipid bilayers which represent the basic building block for biological membranes, nature’s most important interface.
As in many complex fluids and biomolecular assemblies, functionally relevant states and properties depend intricately on hydrated environmental conditions, and the parameters of the aqueous solvent, for example pH, ionic strength and molecular concentrations. It is precisely for that reason that X-ray and neutron diffraction, which are compatible with these environmental conditions, have become such invaluable tools to study so-called soft matter or complex fluids samples. This is in sharp contrast to electrons, which would enable similar resolution, but necessitate vacuum conditions.
In order to concentrate coherent light onto the sample, it was almost always necessary to work with dry or fixated samples, primarily for dose issues and radiation damage. Therefore one of the strongest advantages of the X-ray probe was lost, and the native and functional structure of most complex fluids samples was again inaccessible.
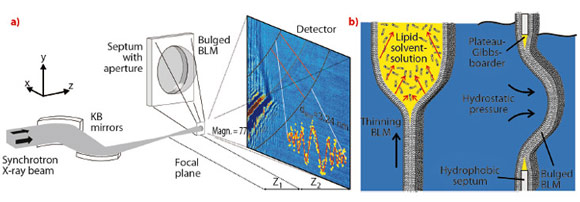
We now report the first X-ray imaging of a hydrated lipid membrane in solution at its native thickness of several nanometres corresponding to two molecular layers, the so-called lipid bilayer. We were even able to witness the formation of a bilayer from two lipid monolayers in a micro-fluidic device. This has become possible by using a particularly dose efficient approach based on free space X-ray propagation, and an analysis strategy which is a combination of near-field diffraction and imaging. A schematic of the experimental setup at ID22NI is shown in Figure 69a. Along the direction perpendicular to the interface, we were able to resolve thickness and density of the membrane derived from the image of its contour.
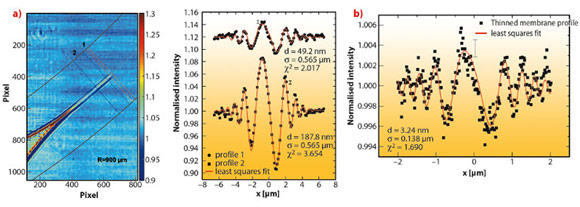
We studied a single freely-suspended lipid bilayer using a well-known setup of membrane electrophysiology, the so-called black lipid membranes (BLM), see Figure 69b, as an established model system in membrane biophysics. The bilayer was spanned in between two separated, aqueous compartments, allowing studies of functional transport across the bilayer at controlled compositional and environmental parameters, such as protein concentration, ionic strength, pH, and electrical field. The membrane is bulged by hydrostatic pressure, and is placed a few millimetres behind the focal plane of a highly efficient and partially coherent elliptical mirror system developed at ESRF. This quasi-spherical illumination leads to a magnified hologram of the sample which is then recorded a few metres behind the sample by the fast read out low noise detector system (FReLoN) devised at ESRF. The Fresnel oscillations in this hologram, corresponding to the native lipid bilayers with a thickness of less than d = 5 nm, were recorded and analysed with respect to the local structure at molecular length scales, see Figure 70a and b.
Straightforward extensions of this approach should enable the extraction of the local interface density profiles of membranes and membrane based materials. More generally, it will allow structure analysis of soft matter interfaces at near-molecular resolution under hydrated conditions, closing the gap between conventional scattering studies on the one hand, carried out over large ensembles and conventional microscopy studies on the other hand. Combining advantages concerning resolution, interaction volume and complexity of the system studied, the experiment paves the way for the controlled investigation of complex biochemical and physical phenomena, particularly soft interfacial phenomena, such as the formation of bilayers, thinning or bulging, as well as in membrane fusion, down to the length scale of a few nanometres.
Principal publication and authors
A. Beerlink (a,b), S. Thutupalli (c), M. Mell (a,d), M. Bartels (a), P. Cloetens (e), S. Herminghaus (c) and T. Salditt (a), Soft Matter 8, 4595-4601 (2012).
(a) Institut für Röntgenphysik, Georg-August-Universität Göttingen (Germany)
(b) current address: Deutsches Elektronen-Synchrotron, PETRA III, Hamburg (Germany)
(c) Max-Planck-Institute for Dynamics and Self-Organization, Göttingen (Germany)
(d) current address: Mechanics of Biological Membranes and Biorheology, Departamento de Quimica Fisica I, Universidad Complutense, Madrid (Spain)
(e) ESRF