- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- X-ray imaging
- Translocation of TiO2 nanoparticles from root to shoot in cucumber plants
Translocation of TiO2 nanoparticles from root to shoot in cucumber plants
The ever increasing use of nanoparticles has raised concerns about the consequences of their release into the environment. TiO2 nanoparticles have been released into the environment by a variety of applications [1]. A few studies have shown that TiO2 nanoparticles accumulate in roots and can affect plants in various ways and one report by Larue and coworkers [2] described the presence of TiO2 nanoparticles in the leaves of a grass plant. In the latter study, synchrotron micro X-ray fluorescence (µ-XRF), combined with X-ray absorption near edge structure (XANES), was used to map the Ti elemental distribution and oxidation states in the roots and leaves of wheat (Triticum aestivum) treated with different size TiO2 nanoparticles in anatase and rutile forms. The researchers found the Ti species within the roots and leaves was TiO2 nanoparticles, with no changes in crystalline structure.
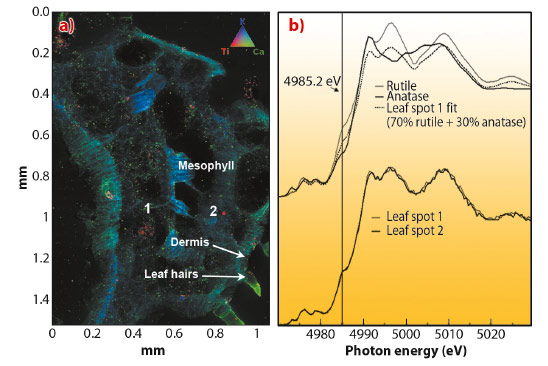
We used µ-XRF combined with µ- XANES at beamline ID21, to study the distribution and speciation of Ti in vegetative tissues of cucumber (Cucumis sativus). Plant sample spectra were compared to spectra of reference materials TiO2 anatase and TiO2 rutile nanoparticles. Cucumber plants were hydroponically grown for 15 days in nutrient solution supplemented with TiO2 nanoparticles (anatase 82%, rutile 18%) at 500 mg L-1. µ-XRF images of transverse sections of TiO2 NP treated roots showed Ti in the epidermis and vascular tissues. In addition, µ-XANES spectra of Ti in the epidermis and xylem resembled the crystalline phase of the anatase TiO2 reference material. However, in the root phloem, the µ-XANES showed the presence of TiO2 mostly in the rutile crystalline phase. As phloem sap plays an important role in fruit formation, the aerial part of cucumber plants was screened for the presence of TiO2. The µ-XRF image of a transverse sectioned cucumber leaf showed the presence of Ti in the central vein of the leaf (Figure 64a, red colour). µ-XANES spectra of red spots from Figure 64a showed that most of the Ti in the leaf vein had the same spectral signature as the rutile TiO2 nanoparticles reference material (Figure 64b).
 |
|
Fig. 64: a) Tricolour micro-XRF map of a transverse section of cucumber leaf treated for 15 days with 500 mg L-1 TiO2 nanoparticles. Colours: red represents titanium, green for calcium, and blue for potassium. b) Micro-XANES spectra of reference materials and spot 1 and 2 from (a). |
Trichomes are structures that protect plants from heat and water loss and they are also involved in heavy metal detoxification [3]. Most of the Cucurbita plants have trichomes in the aerial parts. We analysed leaf trichomes of cucumber plants treated with TiO2 nanoparticles. µ-XRF images showed Ti in the base and body of the trichomes, while the µ-XANES spectra showed that Ti was present mostly in the rutile TiO2 NP crystalline phase. This suggests that leaf trichomes could be a possible sink or excretory system for Ti in cucumber plants.
In conclusion, the µ-XRF and µ-XANES analyses of root and leaf transversal sections have shown that the TiO2 nanoparticles can be absorbed by the roots and translocated to the aboveground plant parts in cucumber. It is noteworthy that cucumber plants were exposed to anatase/rutile mixture of TiO2 nanoparticles, but anatase remained preferentially in the root, while the rutile phase was mainly found in the aerial tissues. This could be a new biological way to separate anatase and rutile. This work also suggests that the TiO2 nanoparticles could be stored in cucumber fruits, entering the food chain and the next plant generation.
Principal publication and authors
A.D. Servin (a,b), H. Castillo-Michel (c), J.A. Hernandez-Viezcas (a,b), B. Corral Diaz (a), J.R. Peralta-Videa (a,b) and J.L. Gardea-Torresdey (a,b), Environ. Sci. Technol. 46, 7637-7643 (2012).
(a) Chemistry Department, The University of Texas at El Paso (USA)
(b) University of California Center for Environmental Implications of Nanotechnology (USA)
(c) ESRF
References
[1] L. Windler and B. Nowack, Environ. Sci. Technol. 46, 8181-8188 (2012).
[2] C. Larue and M. Carriere, Sci Total Environ. 431, 197-208 (2012).
[3] M. Isaure and A. Manceau, Geochim. Cosmochim. Acta 74, 5817-5834 (2010).



