- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Structure of materials
- On the origin of the hydrophobic water gap
On the origin of the hydrophobic water gap
The density deficit of water at hydrophobic interfaces, frequently called the hydrophobic gap, has been the subject of numerous experimental and theoretical studies in the last decade. Recent experiments give values for the interfacial depletion that consistently correspond to less than a monolayer of water. The main question that remained unanswered is its origin and how it is affected by the chemistry and molecular geometry of a particular hydrophobic coating [1].
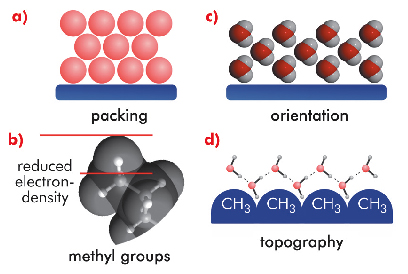
Figure 38 shows several structural features, currently under discussion as possible contributions. Universal packing effects (a) adjacent to a hard wall can affect the liquid structure causing an oscillatory profile with a reduced average density near hydrophobic interfaces. For alkanes, the hydrogen atoms in terminal methyl groups are essentially invisible to X-rays but occupy some space (b). X-ray scattering techniques cannot distinguish this generic volume effect from depletion layers caused by interfacial water. Experiments and simulations indicated that the water molecules next to a hydrophobic interface are partially oriented (c). The hydrogens preferentially point towards the interface, resulting in a geometrically induced local electron depletion. Hydrogen bonds in water arrange more readily around interfacial protrusions than at flat surfaces (d). Thus, the nanoscopic topography can influence the magnitude of the density depletion. In addition to these structural effects, density fluctuations of the liquid result in a reduced contact density, which is most pronounced for very weak water-surface attractions.
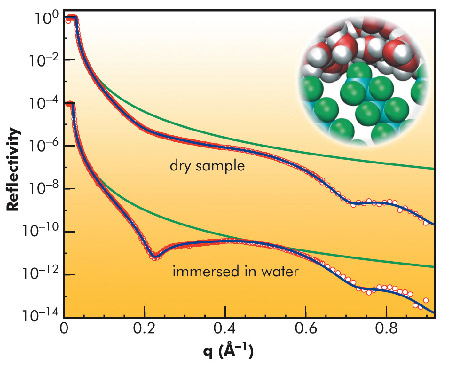
So far, the majority of experimental and numerical studies used aliphatic hydrocarbons as generic hydrophobic model surfaces. To elucidate how the underlying mechanisms affect the extent of the interfacial depletion we carried out a combined high-energy X-ray reflectivity (XRR) and molecular dynamics simulation study of water adjacent to a perfluorinated hydrophobic surface with a spatial resolution on the molecular scale. Comparison of the computational results with the analysis of the XRR data (Figure 39) measured at the high-energy micro-diffraction setup at ID15A revealed the influence of the different contributions to the observed interfacial depletion.
 |
|
Fig. 38: Sketch illustrating various structural features that could contribute to the observed density depletion at hydrophobic interfaces. |
Atomistic simulations of realistic molecularly packed surfaces and XRR on hydrocarbon [2] and perfluorinated self-assembled monolayers consistently confirmed the presence of interfacial depletion with layer thicknesses of at most 2.1 Å. Since the water depletion on perfluorinated substrates is comparable in size, but for which no effects from terminal methyl groups are present, then the methyl groups cannot solely explain the observed gap. Molecular dynamics (MD) simulations showed a broad orientation distribution of interfacial water molecules that is not influenced by the different polarity of the CH3 and CF3 head groups. This suggests that the share from orientational effects of the strongly fluctuating fluid adjacent to a hydrophobic solid is only a minor contribution. In contrast, the surface morphology strongly affects the interfacial depletion and can even be related in a counterintuitive way to the contact angle, commonly used as a macroscopic measure to quantify hydrophobicity. This is of particular importance for more complex soft and biological surfaces that tend generally to show strong corrugations on the length scale of a few angstroms. In addition, there are contributions from molecular packing and capillary wave-like fluctuations that are generic to all fluid interfaces with comparable solid–liquid and liquid–liquid interactions.
 |
|
Fig. 39: X-ray reflectivity from a perfluorinated self-assembled monolayer in air and with the sample immersed in degassed water. Green lines give the Fresnel reflectivity RF of an ideal silicon substrate. Blue lines are best fits to the experimental data. The inset shows a snapshot of an MD simulation of the self-assembled monolayer in contact with water. |
The complex interplay between surface chemistry and topography precludes the existence of a universal relation between the macroscopic contact angle and the nanoscopic water depletion. Thus, there is no clear-cut distinction between hydrophobic and nonhydrophobic contributions to the magnitude and extent of the water gap. However, the combined MD simulation and X-ray reflectivity study for two hydrophobic model interfaces provide typical values that can serve as estimates for many complex interfaces.
Principal publication and authors
M. Mezger (a,b), F. Sedlmeier (c), D. Horinek (c), H. Reichert (a,d), D. Pontoni (d) and H. Dosch (a), J. Am. Chem. Soc. 132, 6735–6741 (2010).
(a) Max-Planck-Institut für Metallforschung, Stuttgart (Germany)
(b) Department of Chemical Engineering, University of California, Berkeley (USA)
(c) Physik Department, Technische Universität München (Germany)
(d) ESRF
References
[1] D. Chandler, Nature 445, 831– 832 (2007).
[2] M. Mezger, H. Reichert, S. Schöder, J. Okasinski, H. Schröder, H. Dosch, D. Palms, J. Ralston and V. Honkimäki, Proc. Natl. Acad. Sci. USA 103, 18401-18404 (2006).



