- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Structure of materials
- Evidence of double-walled Al-Ge imogolite-like nanotubes. A cryo-TEM, AFM and SAXS investigation
Evidence of double-walled Al-Ge imogolite-like nanotubes. A cryo-TEM, AFM and SAXS investigation
Imogolites (OH)3Al2O3Si(OH) are natural minerals discovered in 1962 in Japanese volcanic soils. They are made of a curved gibbsite sheet Al(OH)3 forming a nanotube of 2 nm in diameter. Tetrahedral silicon atoms inside the nanotube are linked to three Al-O-Si bonds. The mismatch between the size of the silicon tetrahedra and the vacant site of the gibbsite sheet leads to the curvature of the tube. The first synthetic imogolite was obtained as early as 1977. Imogolite nanotubes are extremely monodisperse in diameter with lengths up to the micrometre scale. However, early synthesis routes were only effective in very dilute conditions. The difficulty of synthesising large quantities of this mineral has probably hindered the development of industrial applications. Nevertheless, imogolites have been used as an anti-static agent in the formulation of photographic films.
Recently, a new synthetic process with increased synthesis yield was made possible by replacing silicon with germanium. Indeed, we were able to synthesise large quantities of an Al-Ge imogolite analogue. The structure of these analogues has been analysed in detail by small angle X-ray scattering at beamline BM02, by AFM and cryo-TEM microscopy.
 |
|
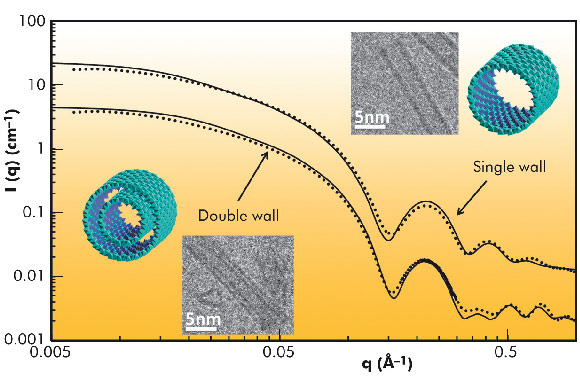
Fig. 30: SAXS curves of 0.5 and 0.25 M Al-Ge imogolite-like nanotubes. Experimental data are plotted using dots, and theoretical scattering curves of the represented structures are plotted using lines. |
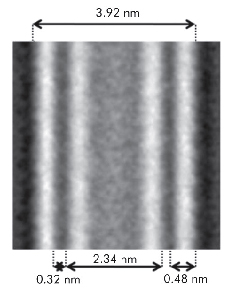
The SAXS analysis unravelled the controversial nature of the so-called proto-imogolite (imogolite precursor) that was poorly known. We demonstrated that the proto-imogolite consists of roof tile shaped 5 nm imogolite nanotube pieces. The curvature of these pieces is less than that of the final tubes [1]. We have also shown that these imogolite analogues have the local structure of imogolite nanotubes, but with two different shapes depending on the synthesis conditions. It is possible to produce single-walled tubes of 3.5 nm in diameter, similar to their natural counterpart, and also double-walled tubes of 4 nm in diameter. Indeed, the SAXS results give distinctly different signals in the large-q range (Figure 30). In the case of single-wall nanotubes, regular oscillations are measured whereas the double-walled structure gives irregular oscillations due to the contribution of the inter wall scattering term. The differences between single and double-wall structures are confirmed by cryo-TEM observations of the samples (Figure 31). Such double-walled imogolite-like nanotubes are reported for the first time and open a new research field. Finally, using time resolved SAXS measurements, we have studied in detail the growth kinetics of the Al-Ge imogolite analogues. We show that the growth is mainly controlled by a reaction limited edge-edge assembly of the nanotubes. The complete simulation of the growth kinetics shows that it could be possible to accelerate considerably the growth kinetics of the imogolite nanotubes [2].
 |
|
Fig. 31: Computer-assisted Cryo-TEM composite image obtained through the alignment and superposition of many nanotubes. The double-wall structure of the 0.25 M Al-Ge imogolite-like nanotubes is clearly observed. |
The precise SAXS measurements made at BM02 allowed us to unravel and quantify a new structure of the Al-Ge imogolite analogues. The current research aims primarily at understanding the mechanisms of formation of these new double-walled nanotubes and also at controlling their organisation. In parallel, a toxicity study is being carried out in our laboratory.
Principal publication and authors
P. Maillet (a), C. Levard (b,e), E. Larquet (c), C. Mariet, O. Spalla (a,e), N. Menguy (c), A. Masion (b,e), E. Doelsch (d,e), J. Rose (b,e) and A. Thill (a,e), J. Am. Chem. Soc. 132, 1208–1209 (2010).
(a) CEA, IRAMIS, Laboratoire Interdisciplinaire sur l’Organisation Nanométrique et Supramoléculaire, Gif-sur-Yvette (France)
(b) UMR 6635 CNRS/Aix Marseille Université, Aix en Provence (France)
(c) Institut de Minéralogie et Physique des Milieux Condensés UMR 7590, CNRS, Université Pierre et Marie Curie, Université Paris Diderot, Institut de Physique du Globe de Paris (France)
(d) CIRAD, UPR Recyclage et risque, Montpellier (France)
(e) iCEINT international Center for the Environmental Implications of Nanotechnology, www.i-ceint.org.
References
[1] C. Levard, J. Rose, A. Thill, A. Masion, E. Doelsch, P. Maillet, O. Spalla, L. Olivi, A. Cognigni, F. Ziarelli and J.Y. Bottero, Chemistry of Materials 22, 2466-2473 (2010).
[2] P. Maillet, C. Levard, O. Spalla, A. Masion, J. Rose and A. Thill, Physical Chemistry Chemical Physics, in press.



