- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structure of materials
- Structure, superconductivity and pressure response of LiFeAs
Structure, superconductivity and pressure response of LiFeAs
High temperature superconductivity has recently been reported in several compounds containing FeAs anti-PbO-type (i.e. antifluorite-type) layers. LaOFeAs was found to superconduct below 26 K when doped with electrons through the substitution of about 10-20% of the oxide ions by fluoride [1]. This critical temperature, Tc, is only exceeded by layered cuprates, some fullerides and MgB2. Subsequently several superconducting compounds containing iron arsenide or phosphide antifluorite-type layers separated by electropositive metal cations, or by metal oxide slabs of varying thickness, have been discovered. The high Tcs (up to 50 K) and critical fields exhibited by these materials suggest that they are unconventional superconductors whose properties cannot be described by existing models of superconductivity such as the BCS theory. The superconducting compositions generally lie close in composition to semimetallic antiferromagnetically ordered phases and it is conjectured that the magnetic properties and the superconducting properties are intimately linked.
Over 40 years ago Juza and Langer [2] reported the existence of a range of phases close to the composition LiFeAs with antifluorite-type FeAs layers, but the electronic properties were not measured. We synthesised stoichiometric LiFeAs samples by the reaction of elemental or binary precursors at 700-800°C and discovered that the samples exhibited bulk superconductivity with Tc as high as 17 K. This discovery of superconductivity in stoichiometric LiFeAs (made in parallel elsewhere [3]) suggested that superconductivity may be ubiquitous in compounds containing FeAs antifluorite layers.
The detailed crystal structure of LiFeAs was probed using a combination of powder neutron diffraction measurements on HRPD and POLARIS at the ISIS facility and powder X-ray diffraction measurements using ID31. The response of the structure to an applied hydrostatic pressure was probed on ID27.
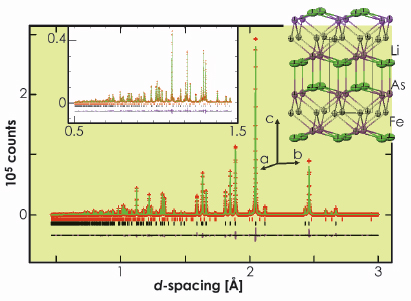
The complementary use of neutrons and X-rays is valuable in this case. There is the possibility of disorder between Fe and Li, and, because of the opposite signs of the neutron scattering lengths of Fe and Li, disorder could mimic the presence of vacancies. Our complementary refinements from HRPD and ID31 (Figure 55) suggested that the superconducting phase is stoichiometric LiFeAs and there is no measurable Li/Fe site disorder. The compound adopts the anti-PbFCl structure type and is composed of an approximately cubic close packed array of As with Fe in half the tetrahedral interstices and Li displaced from the centres of all the octahedral interstices so as to obtain square pyramidal coordination by As (Figure 55). Our combined neutron and X-ray refinement showed that the second Li site postulated by Juza and Langer [2] is unpopulated. These results are consistent with a single crystal diffraction study by Tapp et. al. [3].
 |
|
Fig. 55: The structure of LiFeAs and the refinement against ID31 data. |
LiFeAs has the shortest Fe–Fe distance of any of the iron arsenide superconductors and an FeAs4 tetrahedron that is highly compressed in the basal direction. It also exhibits bulk superconductivity when stoichiometric with Fe formally in the +2 oxidation state, in contrast to the other systems which require doping away from this formal oxidation state in order to realise superconductivity [1]. The relationships between electron count, structural parameters and superconductivity still need to be established. LiFeAs may be an important compound in establishing these relationships because of the differences between it and the other related materials.
 |
|
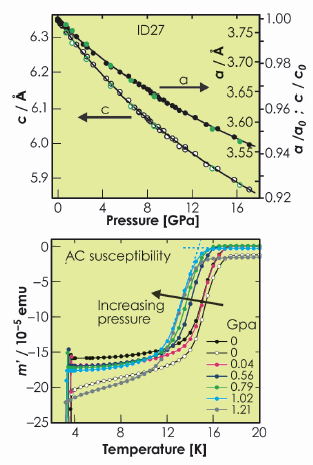
Fig. 56: The pressure response of the lattice parameters refined from ID27 data (upper) and the AC susceptibility (lower). |
Magnetic susceptibility measurements carried out under applied hydrostatic pressure (Figure 56) showed that the superconductivity in LiFeAs is suppressed by the application of pressure. The analysis of powder X-ray diffraction data collected on ID27 from the same sample as the one measured on ID31 shows that LiFeAs is highly compressible with a bulk modulus of 57.3(6) GPa (Figure 56). Refinement of the structural parameters shows that the most compressible part of the structure is the lithium arsenide layer which becomes highly compressed along the stacking direction of the layers. This is presumably because the 5-coordinate site occupied by Li is quite large. In contrast, the FeAs layers become even more compressed in the basal plane and the FeAs4 tetrahedra become even more distorted than at ambient pressure. It has been observed that a high Tc seems to be favoured in systems with very regular FeAs4 tetrahedra, providing the electron count is optimised. The decrease in Tc with increasing distortion of the FeAs4 tetrahedron on LiFeAs is consistent with this observation.
References
[1] Y. Kamihara, T. Watanabe, M. Hirano and H. Hosono, J. Am. Chem. Soc. 130, 3296 (2008).
[2] R. Juza and K. Langer, Z. Anorg. Allg. Chem. 361, 58 (1968).
[3] J.H. Tapp, Z. Tang, B. Lv, K. Sasmal, B. Lorenz, P.C.W. Chu and A.M. Guloy, Phys. Rev. B 78, 060505 (2008).
Principal publications and authors
M.J. Pitcher (a), D.R. Parker (a), P. Adamson (a), S.J.C. Herkelrath (a), A.T. Boothroyd (b), R.M. Ibberson (c), M. Brunelli (d) and S.J. Clarke (a), Chem. Comm., 5918-5920 (2008); M. Mito (b,e), M.J. Pitcher (a), W. Crichton (d), G. Garbarino (d), P.J. Baker (b,c), S.J. Blundell (b), P. Adamson (a), D.R. Parker (a) and S.J. Clarke (a), JACS 131, 2986-2992 (2009).
(a) Department of Chemistry, University of Oxford (UK)
(b) Department of Physics, University of Oxford (UK)
(c) ISIS Facility, STFC-Rutherford Appleton Laboratory (UK)
(d) ESRF
(e) Faculty of Engineering, Kyushu Institute of Technology (Japan)



