- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structure of materials
- New insights into the interface structure of alkanethiol self-assembled monolayers
New insights into the interface structure of alkanethiol self-assembled monolayers
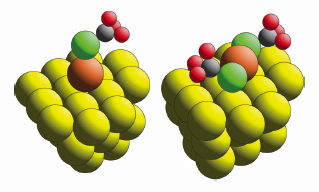
Alkanethiols, CH3(CH2)n-1SH, on the (111) face of gold (typically in the form of an evaporated film) are the archetypal self-assembled monolayer system, and underpin a wide range of applications including sensors, molecular electronics and corrosion protection through functionalisation of the molecular end-group. In all cases the thiol hydrogen is lost by interaction with the surface to produce a thiolate, and the molecule is attached through the S head-group atom. However, even for the simplest system, that of methylthiolate, CH3S–, the structure of the Au/thiol interface remains in doubt [1]. For many years it was assumed that the S atoms occupied the highest (three-fold) coordination ‘hollow’ sites on the hexagonal close-packed outermost surface layer of Au atoms, and subsequent theoretical total energy calculations appeared to support this view, or favoured occupation of two-fold coordinated ‘bridging’ sites. However, two more recent independent experimental studies concluded that the S head-group atom actually bonds atop a single Au surface atom. It is now believed that this apparent inconsistency of theoretical and experimental studies is due to an incorrect underlying assumption of all of the earlier theoretical studies – namely that the Au(111) surface is structurally unchanged by the adsorption. New experiments indicate that the thiolate species are actually bonded to Au adatoms, and that the ‘self-assembly’ in these systems is of Au-thiolate moieties, rather than the thiolate molecules alone. There are, however, two distinctly different adatom models that have been proposed on the basis of previous experiments, namely an Au-adatom-monothiolate, in which the S sits atop a single Au adatom in a hollow site of the underlying surface, and an Au-adatom-dithiolate, in which the Au adatom occupies a bridging site, and the two attached thiolate species occupy adjacent atop sites on opposite sides of the Au adatom (Figure 26).
 |
|
Fig. 26: The two alternative Au-adatom-thiolate models for methylthiolate on Au(111). |
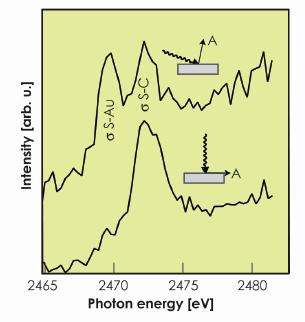
In an attempt to resolve this question of which adatom model is correct, we have used sulphur K-edge near-edge X-ray absorption spectroscopy (NEXAFS) at beamline ID32. Conventionally, the main peaks seen in NEXAFS from molecular adsorbates are assigned to intramolecular scattering resonances. However, aided by theoretical simulations of the spectra, we have shown that, for this adsorption system, one of the two dominant NEXAFS peaks is due to excitation to a ![]() -symmetry S-C intramolecular resonance, but the other involves excitation to a s-symmetry S-Au resonance (Figure 27). The dependence of the amplitude of this latter feature on the direction of the polarisation vector of the incident X-radiation therefore provides a direct indication of the direction of the associated S-Au bond. The data show this bond to be perpendicular to the surface, consistent with atop site adsorption. While both Au adatom structural models have the S head-group atom atop a surface Au atom, in the Au-adatom-dithiolate model the S atom also has an Au atom (the adatom) to one side, creating an additional S-Au bond essentially parallel to the surface. The fact that the NEXAFS resonance assigned to S-Au scattering is only seen under conditions consistent with the S-Au bond being perpendicular to the surface would therefore seem to exclude this model. Further theoretical modelling, however, shows that this is not the case. Specifically, the fact that the Au adatom in this model is bonded to two S atoms causes an energy shift in the NEXAFS peak associated with the parallel S-Au bond such that it overlaps the intramolecular s S-C peak. As such, although the NEXAFS data provide further support for the local atop site of the S head-group atom, they fail to distinguish clearly the two alternative Au-adatom-thiolate reconstruction models.
-symmetry S-C intramolecular resonance, but the other involves excitation to a s-symmetry S-Au resonance (Figure 27). The dependence of the amplitude of this latter feature on the direction of the polarisation vector of the incident X-radiation therefore provides a direct indication of the direction of the associated S-Au bond. The data show this bond to be perpendicular to the surface, consistent with atop site adsorption. While both Au adatom structural models have the S head-group atom atop a surface Au atom, in the Au-adatom-dithiolate model the S atom also has an Au atom (the adatom) to one side, creating an additional S-Au bond essentially parallel to the surface. The fact that the NEXAFS resonance assigned to S-Au scattering is only seen under conditions consistent with the S-Au bond being perpendicular to the surface would therefore seem to exclude this model. Further theoretical modelling, however, shows that this is not the case. Specifically, the fact that the Au adatom in this model is bonded to two S atoms causes an energy shift in the NEXAFS peak associated with the parallel S-Au bond such that it overlaps the intramolecular s S-C peak. As such, although the NEXAFS data provide further support for the local atop site of the S head-group atom, they fail to distinguish clearly the two alternative Au-adatom-thiolate reconstruction models.
 |
|
Fig. 27: Sulphur K-edge NEXAFS recorded at grazing (10°) and normal incidence from an Au(111)/methylthiolate surface. |
Additional information in the S and C atoms sites was also obtained from normal-incidence X-ray standing wave (NIXSW) measurements, providing further confirmation of the atop location of the S atom, but also indicating that the C atoms lie above hollow sites. The S-C bond orientation on the surface implied by this information does appear to favour the monothiolate adatom model.
Reference
[1] D.P. Woodruff, Phys. Chem. Chem. Phys. 10, 7211-7221 (2008).
Principal publication and authors
A. Chaudhuri (a), M. Odelius (b), R.G. Jones (c), T.-L. Lee (d), B. Detlefs (e) and D.P. Woodruff (a), J. Chem. Phys. 130, 124708 (2009).
(a) University of Warwick, Coventry (UK)
(b) Stockholm University, Stockholm (Sweden)
(c) University of Nottingham, Nottingham (UK)
(d) Diamond Light Source Ltd, Didcot (UK)
(e) ESRF



