- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Materials science
- Polyamorphic transition, charge-density waves and superconductivity in sulphur
Polyamorphic transition, charge-density waves and superconductivity in sulphur
Elemental sulphur, a yellow insulating mineral, is composed of crown-like 8-member ring molecules that form a complex crystal structure. Compression has a dramatic effect on these molecules: they break apart forming various chain and ring structures on pressure increase [1] changing colour to red at around 8 GPa. By compressing sulphur at low temperatures, where the phase transitions are kinetically inhibited, we find that the ambient pressure phase of sulphur with S8 molecules persists in a metastable form to much higher pressures becoming amorphous above 40 GPa. Sulphur becomes metallic and superconducting above 90 GPa with Tc = 10 K [2] forming an incommensurately modulated crystal structure at 300 K [3]. By studying the crystal structure of metallic sulphur at low temperatures close to the superconducting transition, we find a charge-density wave (CDW) instability, responsible for the structural modulation, to be in competition with the superconducting state.
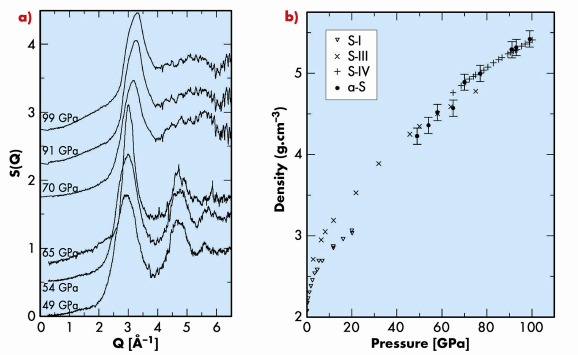
Using high-pressure X-ray powder diffraction techniques on beamline ID09A with a liquid He cryostat, we compressed the S-I phase of sulphur at low temperatures observing a transition to an amorphous state (a-S) at pressures above 40 GPa. A change in the diffraction pattern of a-S is seen at 65 GPa and 80 K (Figure 35a), signalling a transformation from one amorphous form to another. Diffraction-based direct density measurements give a 7% density discontinuity (Figure 35b) corresponding to a transition from a low-density amorphous (LDA) to a high-density amorphous (HDA) form. The similarities between the densities and local structures of LDA and HDA sulphur with those of phases S-III (square chains) and S-IV (incommensurately modulated) are striking, suggesting a nanocrystalline nature instead of truly amorphous form of a-S. Further compression of the high-density a-S resulted in its recrystallisation into phase S-IV at 99 GPa and 40 K.
 |
|
Fig. 35: a) Structure factors of low-density amorphous form of sulphur (49 and 54 GPa and 175 K, 65 GPa and 80 K) and high-density amorphous form of sulphur (70, 91 and 99 GPa and 40 K). b) Pressure dependence of density of a-S compared to crystalline phases. |
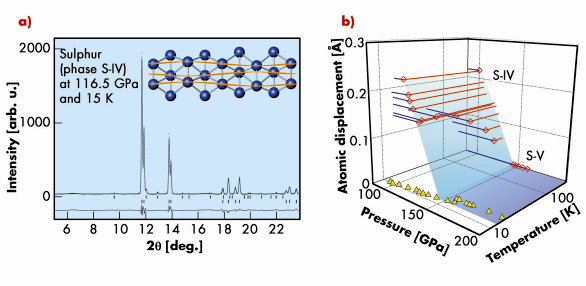
We observed sharp modulation reflections in the S-IV phase, characteristic of a long-range ordered CDW, and traced their positions and intensities as a function of pressure and temperature. From Rietveld refinement of diffraction patterns (Figure 36a) at each P-T point we obtained the amplitude of the atomic displacements due to modulation in the P range from 100 to 165 GPa and T range from 300 down to 15 K, close to the superconducting transition temperature (Figure 36b). The amplitude of the structural modulation (Figure 36a, inset) decreases with pressure increase, which correlates with the gradual increase in Tc from 10 to 14 K [2]. The modulation disappears at 153 GPa, signalling the suppression of the CDW, leading to the enhancement of Tc from 14 to 17 K at this pressure.
 |
|
Fig. 36: a) X-ray diffraction profile of S-IV and a Rietveld refinement on the basis of the incommensurately modulated structure. The inset shows the crystal structure of S-IV. b) Observed maximum atomic displacement in S-IV and S-V as a function of pressure and temperature, shown as open diamond symbols. Triangles show Tc from Ref. [2]. |
In conclusion, we have shown that sulphur exhibits a pressure-induced amorphisation at low temperatures and pressures above 40 GPa, with a further transition from a low-density to a high-density amorphous form at 65 GPa. Present work has extended the pressure range of the direct density measurements of amorphous materials in the diamond anvil cells by a factor of 100. At a higher pressure of 100 GPa, sulphur crystallises into an incommensurately modulated structure, which is formed because of a CDW instability. Sulphur now represents a unique example of an elemental metal undergoing a CDW transition within a superconducting state, and joins a large group of complex materials showing an enhancement of Tc upon pressure-induced suppression of a CDW.
Principal publications and authors
C. Sanloup (a), E. Gregoryanz (b), O. Degtyareva (b), and M. Hanfland (c), Phys. Rev. Lett. 100, 075701 (2008); O. Degtyareva (b), M.V. Magnitskaya (d), J. Kohanoff (e) G. Profeta (f) S. Scandolo (g) M. Hanfland (c), M.I. McMahon (b) and E. Gregoryanz (b), Phys. Rev. Lett. 99, 155505 (2007).
(a) School of Geosciences and Centre for Science at Extreme Conditions, University of Edinburgh (UK)
(b) SUPA, School of Physics and Centre for Science at Extreme Conditions, University of Edinburgh (UK)
(c) ESRF
(d) Institute for High Pressure Physics, Russian Academy of Sciences, Troitsk (Russia)
(e) Atomistic Simulation Centre, Queen’s University Belfast (UK)
(f) CNISM-Dipartimento di Fisica, Universita` degli Studi di L’Aquila (Italy)
(g) The Abdus Salam International Centre for Theoretical Physics (ICTP) and INFM/CNR ‘‘Democritos’’ National Simulation Centre, Trieste (Italy)
References
[1] O. Degtyareva et al., J. Chem. Phys. 126, 084503 (2007).
[2] E. Gregoryanz et al., Phys. Rev. B 65, 064504 (2002).
[3] C. Hejny et al., Phys. Rev. B 71, 020101(R) (2005).



