- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- X-ray imaging and optics
- Carbon nanotubes in macrophages: imaging and chemical analysis by synchrotron X-ray fluorescence microscopy
Carbon nanotubes in macrophages: imaging and chemical analysis by synchrotron X-ray fluorescence microscopy
The human health impact associated with carbon nanotubes (CNT) is a critical issue considering their numerous potential applications. Basic research on interactions between carbon nanotubes and cells is needed, especially for their localisation within cells. A second requirement is to have information about cell modifications after contact with carbon nanotubes. Both points can be addressed using synchrotron-based X-ray fluorescence microscopy (µXRF), used here for the first time to study carbon nanotube-cell interactions.
In this study, we consider murin macrophages exposed to unpurified or purified single-walled (SW) CNT or to multi-walled (MW) CNT, the choice of macrophage cells being dictated by their key role in host responses to exogenous agents (Figure 148). Two-dimensional fluorescence scans have been performed on cryofixed samples, on beamline ID21, with a beam size of 1.5 µm x 0.5 µm and with incident X-ray energy of 7.2 keV. This energy was chosen to excite the K-lines of elements of atomic number up to Z = 26, corresponding to iron, which is the catalyst of the growth of the studied nanotubes. Iron particles are found inside MWCNT or bound to SWCNT bundles, and can therefore be used as a nanotube tracer inside cells. The purification process considered in SWCNT samples leads to a decrease of the amount of iron from 20 wt% to 2wt% in unpurified and purified samples, respectively.
 |
|
Fig. 148: Artistic view of a macrophage cell engulfing a bundle of single-walled carbon nanotubes. Iron catalytic particles, surrounded by carbon shells and bound to nanotubes, are shown in orange (not to scale). |
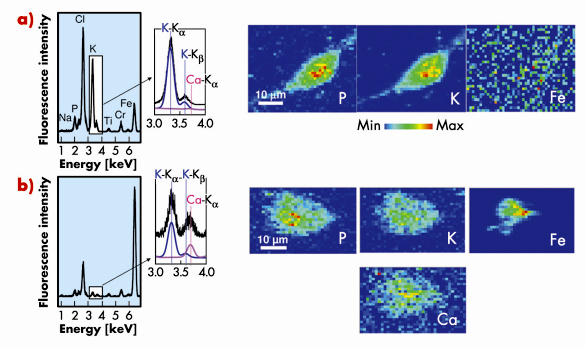
Figure 149a shows fluorescence spectra and elemental maps of an unexposed cell obtained using µXRF. Phosphorus and potassium are constitutive elements of cells and their spatial repartition delimitates the cell shape. The P and K-rich zones in the central part of the cell can be attributed to the nucleus. Iron signal is found to present a rather homogeneous distribution and is attributed to experimental background.
Considering carbon nanotube-exposed cells, iron maps reveal one or several iron-rich zone(s) inside or close to the cell contours (see Figure 149b). These zones correspond to CNT localisation. Iron-based catalyst particles could even be detected in the case of cell exposure to purified SWCNT, for which iron amount is minimised, thus highlighting the high sensitivity of the µXRF method. In the case of macrophages exposed to unpurified SWCNT, an additional calcium signal following the cell-shape can be evidenced (Figure 149b) although no calcium signal is detectable in unexposed cells. This finding of extra-cellular calcium uptake by exposed cells could be of strong importance in the perspective of understanding the toxicological mechanisms of CNT [1,2]. Moreover, pharmacological assays with calcium chelators and inhibitors, performed following this µXRF experiment, point toward the key role of calcium in carbon nanotube cytotoxicity.
 |
|
Fig. 149: X-ray fluorescence spectra integrated over the whole scanned areas of a typical control cryo-fixed macrophage a) and of a macrophage exposed for 24h to an unpurified SWCNT suspension at 100 µg/mL b). Enlarged areas around the positions of the K |
Calcium uptake was shown in part of the cells exposed to unpurified SWCNT or MWCNT but in none of those exposed to purified SWCNT. Chemical or physical parameters of CNT, such as their catalyst content (higher in unpurified SWCNT and MWCNT than in purified ones) or their surface properties (which are modified during purification process) could be crucial determinants in their toxicity. Effect of the nanotube aspect ratio and length may also be relevant. ESRF capabilities could allow one to go further in the understanding of carbon nanotube-cell interactions: micro-XANES could be used to identify putative changes in the chemical state of the iron nanoparticles during cell contact and nano-XRF tomography could give new information about CNT toxicity at the sub-cellular level.
Principal publication and authors
C. Bussy (a,b), J. Cambedouzou (a), S. Lanone (b), E. Leccia (a), V. Heresanu (a), M. Pinault (c), M. Mayne-l’Hermite (c), N. Brun (a), C. Mory (a), M. Cotte (d), J. Doucet (a), J. Boczkowski (b), and P. Launois (a), Nano Letters 8, 2659 (2008).
(a) Laboratoire de Physique des Solides, CNRS-Université Paris-Sud 11, Orsay (France)
(b) Institut Mondor de Recherche Biomédicale, INSERM-Université Paris Val de Marne/Paris 12, Créteil (France)
(c) Laboratoire Francis Perrin, CEA-CNRS, Saclay (France)
(d) ESRF
References
[1] D.M. Brown, et al., Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L344 (2004).
[2] K. Donaldson, et al., Free Radical Biol. & Med., 34, 1369 (2003).
Acknowledgement
The authors acknowledge financial support from the Agence Nationale de la Recherche and C'Nano Ile de France.



