- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Structural biology
- Structure-function analysis of a bacterial tyrosine kinase involved in capsule assembly
Structure-function analysis of a bacterial tyrosine kinase involved in capsule assembly
In bacteria, some polysaccharides co-polymerases involved in capsule synthesis have been identified as protein-kinases belonging to a new family of tyrosine-kinases and called BY-kinases. They show no sequence similarity with eukaryotic protein-kinases. BY-kinases contain Walker A and B nucleotide-binding motifs characteristic of the so-called P-loop proteins. They undergo autophosphorylation on a C-terminal tyrosine cluster and further phosphorylate endogenous protein substrates. In proteobacteria, BY-kinases are transmembrane proteins containing a periplasmic domain and a cytoplasmic catalytic domain. In firmicutes, BY-kinases are encoded by two adjacent genes and split in two polypeptides, which correspond each to one of these two domains.
In this work, we focused on the BY-kinase CapA/CapB of Staphylococcus aureus, a pathogen responsible for a diverse spectrum of animal and human diseases that predominates among clinical isolates. The interaction between the cytoplasmic protein-kinase CapB and the C-terminal juxtamembrane fragment of the membrane protein CapA (CapA-Ct) is required to promote CapB activity. Here we present the crystal structures of the chimerical protein CapA-Ct/CapB (CapAB) and of its inactive P-loop mutant CapAB(K55M).
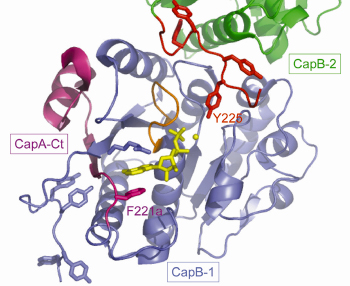
Non-denaturing electrophoresis and mass spectrometry analysis demonstrated that the purified CapAB protein is autophosphorylated. It crystallised in 20% PEG 1000 and high-resolution diffraction data (1.8 Å) were collected at beamline ID23-2. The CapAB structure was solved by molecular replacement using the P-loop ATPase MinD as starting model. It is monomeric, with CapB harbouring the typical α/β-fold of P-loop proteins. The phosphorylated C-terminal tyrosine cluster of CapB is disordered. CapA-Ct forms α-helix followed by a β-strand that completes the central β-sheet of CapB. CapA-Ct is involved in nucleotide binding via its penultimate residue Phe221a, which participates in a stacking interaction with the purine ring of the ADP molecule found close to the P-loop of CapB (Figure 72).
 |
|
Fig. 72: A view of the active site of the S. aureus BY-kinase mutant CapAB(K55M). The P-loop of the blue CapB-1 is shown in orange with bound ADP-Mg in yellow. The CapA-Ct activator domain is shown in magenta. F221a involved in nucleotide binding is highlighted as are the phosphorylatable Y225 from the C-terminal tyrosine cluster (red) of a neighbouring CapB-2 subunit (green). |
This explains the stimulatory effect of CapA-Ct on the kinase activity of CapB.
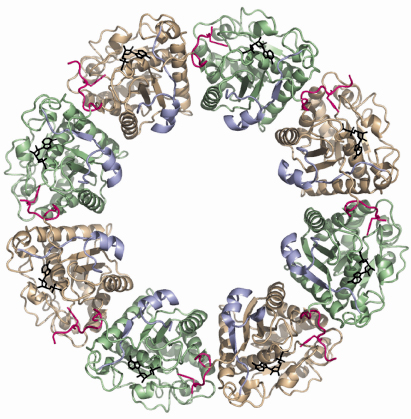
The structure of the inactive P-loop mutant CapAB(K55M) was solved at beamline ID29. It presents a subunit fold highly similar to that of the CapAB with an rmsd of 0.67 Å over 241 aligned residues. The major difference concerns its quaternary structure. Surprisingly, the unphosphorylated protein forms a ring-shaped octamer (Figure 73) with the C-terminal tyrosine cluster from each molecule inserted into the active site of a neighbouring one (Figure 72). The last tyrosine residue in the cluster, Tyr225, points towards the phosphate tail of the bound ADP and interacts with the catalytic Asp79 that is in a position to deprotonate the Tyr225 hydroxyl group, a prerequisite for its phosphorylation.
 |
|
Fig. 73: Ring-shaped octamer of the S. aureus BY-kinase mutant CapAB(K55M). CapB subunits are alternatively colored green and beige with the C-terminal tyrosine clusters highlighted in pink. The CapA-Ct activator domains are shown in blue. Bound nucleotides are shown as black sticks. |
In the octamer, the buried surface areas between neighbouring CapAB subunits (more than 2000 A2) are typical of those required for biological interactions. Moreover, when a comparison is made with the other BY-kinase sequences, the absolute conservation of the residues involved in monomer-monomer interactions clearly demonstrates that the octamer is physiologically important. Our structural analysis clearly shows that BY-kinase autophosphorylation proceeds via an intermolecular mechanism. Our data further suggest that the cytoplasmic kinase domains dissociate from each other upon phosphorylation, inducing conformational changes transmitted to the other components of the capsule synthesis machinery via the associated transmembrane activation domain. In this model, cyclic phosphorylation/dephosphorylation of the BY-kinase seems to be intimately correlated to its polysaccharide co-polymerase activity.
This work represents the basis for structure-based drug design projects aiming at blocking bacterial capsule production. In S. aureus, but also in other bacterial pathogens, capsular polysaccharides are considered as major virulence factors. Considering the idiosyncratic nature of BY-kinases, which are not present in eukaryotic organisms, these enzymes represent promising targets for the design of drugs with limited side effects on host cells.
Principal publication and authors
V. Olivares-Illana (a), P. Meyer (a), E. Bechet (b), V. Gueguen-Chaignon (a), D. Soulat (b), S. Lazereg-Riquier (c), I. Mijakovic (d), J. Deutscher (e), A. Cozzone (b), O. Laprévote (c), S. Morera (a), C. Grangeasse (b) and S. Nessler (a), PLoS Biol. 6, e143 (2008).
(a) LEBS, CNRS UPR 3082, Gif sur Yvette (France)
(b) IBCP, UMR 5086 CNRS, Université de Lyon (France)
(c) ICSN, CNRS UPR 2301, Gif sur Yvette (France)
(d) Center for Microbial Biotechnology, DTU, Lyngby (Denmark)
(e) LMGM, AgroParisTech, CNRS, INRA, Thiverval-Grignon (France)



