- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Structural biology
- Structure of a "bonsai" Ndc80 complex sheds light on the mechanism of chromosome segregation
Structure of a "bonsai" Ndc80 complex sheds light on the mechanism of chromosome segregation
Accurate chromosome segregation is a prerequisite for long-term cell and organism survival. To ensure this, the replicated chromosomes (known as sister chromatids) establish sturdy connections with a structure known as the mitotic spindle. Microtubules, which are polymeric structures based on the assembly of αß-tubulin dimers, are the main component of the spindle. During the process of cell division, chromosomes assemble and expose a microtubule receptor known as the kinetochore. The crucial microtubule receptor at kinetochores is a 4-protein complex known as the Ndc80 complex. Our studies have provided a molecular understanding of the organisation of the Ndc80 complex and of its ability to bind microtubules.
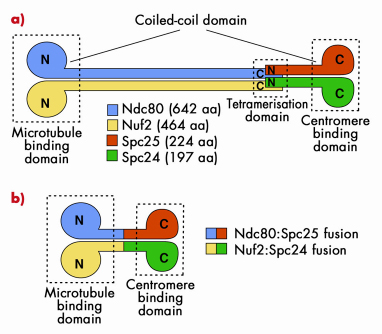
The Ndc80 tetramer is assembled from two dimers, Ndc80:Nuf2 and Spc24:Spc25 (Figure 79a). The topology of interactions of these two dimers in the Ndc80 complex was known from previous studies. Ndc80:Nuf2 have globular N-terminal domains and are predicted to form a long parallel coiled-coil with their C-terminal regions. The Ndc80:Nuf2 coiled-coil ends in a tetramerisation domain where the Ndc80:Nuf2 dimer meets the Spc24:Spc25 dimer. The Spc24:Spc25 dimer contains another dimeric parallel coiled-coil that engages the N-terminal regions of the Spc24:Spc25 subunits, and ends with a globular region. This organisation of the Ndc80 complex results in a very elongated and rather flexible structure, as shown by low-resolution electron micrographs on negatively stained samples. The globular regions at either end of the rod have been shown to mediate microtubule binding and kinetochore localisation.
 |
|
Fig. 79: a) The Ndc80 complex is a tetrameric arrangement of Ndc80, Nuf2, Spc25 and Spc24 subunits. Ndc80:Nuf2 and Spc25.Spc24 form dimeric arrangements containing microtubule-binding and kinetochore-binding domains, respectively. Overall, the complex is dumb-bell shaped consisting of two globular domains separated by a central coiled-coil rod. b) Bonsai version of the Ndc80 complex. The chains have been fused two-on-two and most of the coiled coil domain has been removed. |
Highly elongated and flexible structures are almost invariably difficult to crystallise. Thus, to gain high-resolution structural insight into the Ndc80 complex, we made an attempt to simplify its organisation. Because the topology of the Ndc80 complex suggested that the C-terminal moieties of the Ndc80:Nuf2 subunits are close to the N-terminal moieties of the Spc24:Spc25 subunits, we tried to fuse the chains two on two to create a dimeric strucure (Figure 79b). We then removed most of the intervening coiled-coil region to create a Bonsai Ndc80 complex containing only the “leaves” (the globular domains of Ndc80 and Nuf2), a small segment of the “trunk” (the coiled-coil), and the “roots” (the globular region of the Spc24:Spc25 dimer. The topology of this construct is shown in Figure 79b. The Ndc80bonsai construct retains the ability to bind microtubules and kinetochores.
 |
|
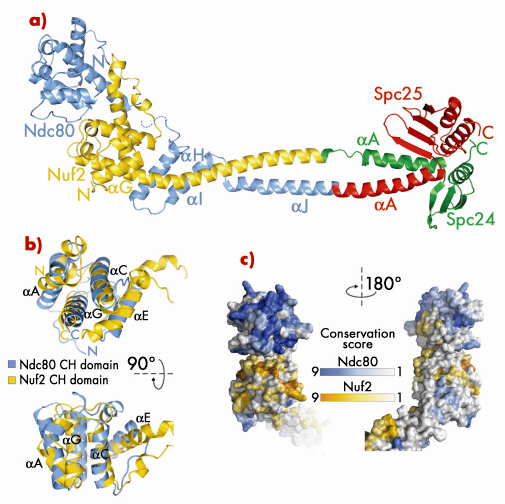
Fig. 80: a) Ribbon model of Bonsai-Ndc80. b) Superposition of the calponin-homology (CH) domains of Ndc80 (cyan) and Nuf2 (yellow). c) Map of evolutionary residue conservation on the surface of the Ndc80 complex identifies a microtubule-binding interface (left). |
Bonsai-Ndc80 proved to be very stable and crystallised readily. Using X-ray diffraction data collected on beamlines BM16 and ID23-1 we were able to determine the 2.8 Å crystal structure of the Bonsai-Ndc80 complex (Figure 80a). The structure revealed that the globular domains of Ndc80 and Nuf2 each contain a calponin-homology (CH) domain (Figure 80b). CH domains have been previously implicated in binding actin or microtubules. In the Ndc80:Nuf2 moiety, the CH domains are tightly packed and form a single large exposed surface. We have carried out an extensive mutagenesis analysis to demonstrate that this surface provides the main microtubule-binding area of the Ndc80 complex. Based on the structure of Bonsai-Ndc80 and of cross-linking and mass spectrometry data, we have generated a model for the full-length Ndc80 complex and revealed the molecular basis of chromosome segregation, a process that is essential for the propagation of genetic information along successive generations. Our current analyses aim at understanding the broader organisation of multiple Ndc80 complexes around microtubules.
Principal publication and authors
C. Ciferri (a), S. Pasqualato (a), E. Screpanti (a), G. Varetti (a), S. Santaguida (a), G. Dos Reis (a), A. Maiolica (a), J. Polka (b), J.G. De Luca (c), P. De Wulf (a), M. Salek (d), J. Rappsilber (e), C.A. Moores (f), E.D. Salmon (b) and A. Musacchio (a), Cell 133, 427 (2008).
(a) Department of Experimental Oncology, European Institute of Oncology, Milan, (Italy)
(b) Department of Biology, University of North Carolina (USA)
(c) Dept of Biochemistry and Molecular Biology, Colorado State University (USA)
(d) Sir William Dunn Pathology School, Oxford (UK)
(e) Wellcome Trust Centre for Cell Biology, University of Edinburgh (UK)
(f) School of Crystallography, Birkbeck College, University of London (UK)



