- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- X-ray absorption and magnetic scattering
- Alkali metal-intercalated fullerenes under high pressure
Alkali metal-intercalated fullerenes under high pressure
Alkali metal-intercalated fullerenes are attractive as precursors for new materials (high-dimensional polymers) exhibiting both superconducting and high hardness properties [1], that can be formed via high-pressure synthesis. Nevertheless, the strong ionic interactions between the alkali atoms and the C60 molecule were predicted to give rise to a deformation of the C60 the isolated icosahedral molecule [2]. This deformation could limit the pressure stability of the molecule. However, no experimental evidence of such deformation has been provided before our experiments. Therefore, exploration of the P-T phase diagram of alkali metal-intercalated fullerenes is of particular interest because of these predictions.
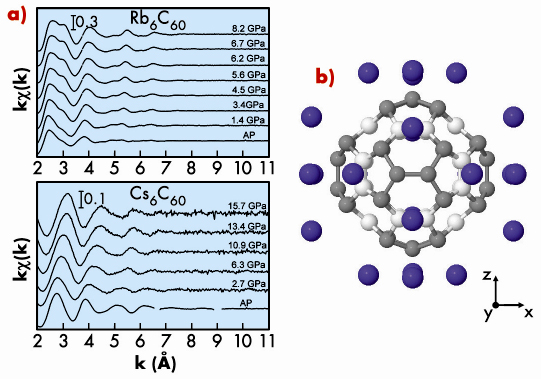
We studied the body-centered cubic (bcc) Rb6C60 (a = 11.54 Å) and Cs6C60 (a = 11.79 Å) systems by employing different complementary techniques: X-ray diffraction (XRD), X-ray absorption fine structure (XAFS) and Raman spectroscopies. XRD (beamline ID27) and XAFS measurements (beamline BM29) were carried out up to 15 GPa at room temperature on both systems. By comparing the compressibility of the total volume obtained by XRD data with the compressibility of the interstitial volume between the C60 and the alkali atoms obtained by XAFS, we observed that its compression is accompanied by a shape-changing deformation under pressure, this is reported in Figure 115.
The distortion of the molecule is probably due to an enhancement of the Coulomb interaction with the surrounding ions, effectively increasing the difference between the distances from each molecule’s centre to those 36 carbons closest to the alkali metals (grey atoms in Figure 115), on one hand, and to those 24 carbon atoms located closest to other C60 molecules (white atoms in Figure 115), on the other hand. This deformation is analogous to pulling the molecule through the three orthogonal axes pointing towards the bcc faces containing the alkali metals.
The deformation of the fullerene molecule is greater (54% at 15 GPa) for Rb than for Cs intercalation, this too confirmed by our ab initio calculations for both systems.
In spite of such slight deformation due to the pressure enhanced Coulomb interaction, Raman measurements performed under pressure on Cs6C60 indicated that the C60 cage is preserved up to a pressure two times higher (45 GPa) than for pristine C60, which was observed to amorphise at 22 GPa.
 |
|
Fig. 115: a) Pressure evolution of the XAFS signal; b) Pressure-induced distortion (amplified by a factor of 30) of the C60 molecule at 15 GPa observed in Rb6C60 and Cs6C60. |
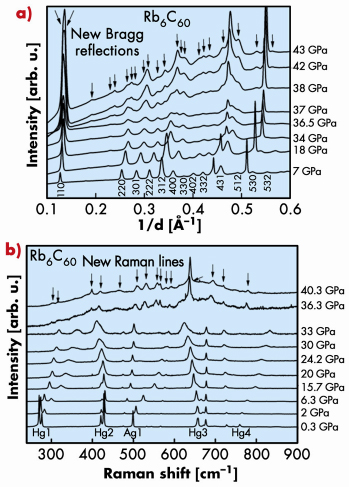
Conversely, XRD, XAS (beamline ID24) and Raman spectroscopy measurements for Rb6C60 under HP-HT conditions showed the existence of a first-order phase transition at around 35 GPa from the bcc towards a hexagonal phase accompanied by a discontinuous decrease of the unit cell volume. Both XRD and Raman data collected under pressure are shown in Figure 116. The lattice parameters of the hexagonal unit cell (a = 8.360 Å and c = 14.830 Å) are compatible with a distance between molecules previously observed in the case of polymerised C60. In addition, in correspondence with the transition, the Raman data show a discontinuous change in the frequency of the intramolecular vibrations. This is probably associated with the breaking of some intramolecular bonds concomitant with the formation of intermolecular covalent bonds, as occurs in cycloaddition processes characteristic of the C60 polymerisation. Hence, we suggest that the hexagonal HP phase could be associated to the formation of 2D polymers in the (001) plane of the hexagonal structure.
 |
|
Fig. 116: a) Pressure evolution of XRD, and b) Raman measurements for Rb6C60. The occurrence of a phase transition at around 35 GPa is indicated by the appearance of new Bragg reflections and Raman lines, respectively. |
The lower pressure stability of the cubic phase (and of the molecular character) observed in Rb6C60 compared to Cs6C60 is concomitant with a more pronounced distortion of the molecule in the first compound compared to the latter. Nevertheless, both fullerides show a much higher pressure stability of the molecular character of the system, compared to the solid C60. We therefore infer that the presence of such ionic host-guest interactions could be responsible for the high pressure stabilisation of the C60 molecules.
Principal publications and authors
R. Poloni (a), G. Aquilanti (b), S. Le Floch (c), M. V. Fernandez-Serra (d), S. De Panfilis (e), P. Toulemonde (f), D. Machon (c), G. Morard (b), D. Martinez-Blanco (g), W. Crichton (b), S. Pascarelli (b), A. San-Miguel (c), Phys. Rev. B 77, 035429 (2008); Phys. Rev. B 77, 205433 (2008).
(a) ICMAB-CSIC, Barcelona (Spain)
(b) ESRF
(c) LPMCN, University of Lyon (France)
(d) Stony Brook, New York (US)
(e) INFM-CNR, Roma (Italy)
(f) Institut Néel, CNRS and UJF, Grenoble (France)
(g) Universidad de Oviedo (Spain)
References
[1] D. Connétable et al., Phys. Rev. Lett. 91, 247001 (2003).
[2] W. Andreoni et al., Phys. Rev. Lett. 68, 823 (1992).



