- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- X-ray absorption and magnetic scattering
- Kinetics and thermodynamics of siderite dissolution at 300 bar between 50°C and 100°C
Kinetics and thermodynamics of siderite dissolution at 300 bar between 50°C and 100°C
Iron-bearing minerals are reactive phases of the subsurface environment. Siderite (FeCO3) is one of the most abundant of these and it plays a major role in the deep geological storage of CO2, both by its presence in the reservoir rock where the injection might be done, and by its own constitution, being a form of precipitated CO2 during its injection in deep aquifers. It is thus of utmost importance to precisely model the stability and dissolution kinetics of siderite under hydrothermal subsurface conditions.
The dissolution of siderite FeCO3 in acidic hydrothermal conditions (50-100°C, 300 bar, 0.1 M HCl) has been studied by X-ray Absorption Spectroscopy. The absorption spectra are the data on which our methodology is based. Taken at the Fe K-edge, and measured on the aqueous solution in contact with the dissolving mineral they allow:
• determination of the dissolution rate of siderite, as a function of time, from the absorption value of the solution.
• determination of the speciation of dissolved iron by comparing the absorption spectra with reference spectra of iron complexes in hydrothermal conditions.
• in situ measurement in a high pressure-high temperature autoclave which permits working with macroscopic samples (siderite samples are millimetric single crystals) + 50 mm3 of solution, [1]). Results are directly related to subsurface CO2 storage conditions.
 |
|
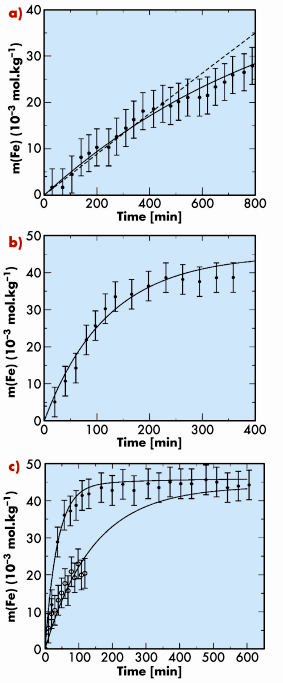
Fig. 123: Iron molality as a function of time for siderite dissolution at 300 bar and 0.1 m HCl at a) 50°C, b) 75°C, c) 100°C. The experimental data points are shown as filled circles (empty circles in c) are for a 1 mol/Kg NaCl solution). The continuous lines are calculated with the Chess software using the dissolution rate constants measured in this study [2]. |
Figure 123 and Figure 124 summarise the data collected in our experiment. Using the molalities of Fe(II) in the aqueous phase (Figure 123), a geochemical model was built using Chess [2] from which kinetics rate constants are derived at each temperature, as well as the activation energy of the system. Thanks to this model, the distribution of iron aqueous species is also calculated, and compared with a very good agreement with the speciation derived from the XANES measurements (Figure 124). This XANES signal is characteristic of the hydration structure of dissolved iron, and it is interpreted thanks to a parallel study where the speciation of iron is determined as a function of temperature, pressure and chloride concentration by EXAFS analysis and XANES ab initio calculations.
 |
|
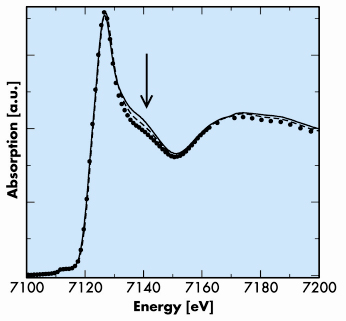
Fig. 124: Comparison of XANES spectra obtained for hydrated Fe2+ (solid line) and at the end of the siderite dissolution experiment at 100°C (dashed lines: without NaCl; filled circles: with 1 mol kg–1 NaCl). The three spectra are very close and mainly differ in the 7130–7150 eV region (indicated by the arrow), with slight differences in the 7170–7200 eV region. All the spectra show the dominance of hydrated Fe2+ species. The differences are interpreted by minor occurrence of FeCl+ and/or FeCl2 species, more significant, as expected, in 1 mol kg–1 NaCl. |
The kinetics rate constants obtained in these closed-reactor pH-varying experiments compare favourably with values from recent experiments carried out at constant chemical affinity [3]: this indicates that we can retrieve kinetics parameters from batch micro-reactor experiments conducted at synchrotron radiation facilities that can be coupled with spectroscopic speciation results. These data are now incorporated in geochemical software modelling fluid-rock interactions under CO2 storage conditions.
Principal publication and authors
D. Testemale (a), F. Dufaud (b), I. Martinez (b), P. Bénezeth (c), J.-L. Hazemann (a), J. Schott (c), F. Guyot (b,d), Chemical Geology, doi:10.1016/j.chemgeo.2008.08.019 (2008).
(a) Institut Néel and FAME/ESRF, CNRS, Grenoble (France)
(b) CO2 storage research center. Institut de Physique du Globe, Paris (France)
(c) Laboratoire de Mécanismes des Transferts en Géologie, CNRS-IRD-OMP, Toulouse (France)
(d) Institut de Minéralogie et de Physique des Milieux Condensés, CNRS-Paris6-Paris7, Paris (France)
References
[1] D. Testemale, R. Argoud, O. Geaymond, J.-L. Hazemann, Rev. Sci. Instrum., 76, 043905 (2005).
[2] J. van der Lee, Technical Report LHM/RD/98/39, CIG, Ecole des Mines de Paris, http://chess.ensmp.fr/
[3] S. V. Golubev, P. Bénézeth, J. Schott, J.-L. Dandurand, A. Castillo, Chem. Geol., Special issue on CO2 geological sequestration, in press.



