- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- High resolution and resonance scattering
- Exploring the dynamic platinum structure during CO oxidation
Exploring the dynamic platinum structure during CO oxidation
The oxidation of carbon monoxide is one of the most intensively studied reactions in the field of catalysis because of its role in the purification of feed gas for fuel cells and automotive exhaust gas. Knowledge of the structure of the catalytically-active sites in these solid catalysts is essential to understand the functioning of solid catalysts and chemical processes. Despite the large effort in research in this field, fundamental questions about the active species and reaction mechanism remain disputed. Ertl and co-workers showed that on single crystals under low-pressure conditions, varying reconstruction of the platinum surface, which occurs after adsorption of carbon monoxide, leads to carbon monoxide-rich and oxygen-rich domains that have different reaction rates [1]. High-pressure experiments on single crystals however, suggested that oxidised platinum is the active phase [2]. In the work presented here, we have bridged both the pressure and materials gaps by determining the structure of a supported platinum catalyst during oxidation of carbon monoxide under real conditions. Bridging the pressure and materials gaps is essential to translate results from single crystal studies and understand catalytic processes under realistic conditions. This was achieved by combining in situ, time-resolved, and high-energy resolution fluorescence X-ray spectroscopy (HERFD XAS) [3,4] with kinetic measurements. HERFD yields sharper spectral features because the instrumental energy broadening is below the width due to the core-hole lifetime, which enables detection of considerably more spectral detail. HERFD XAS were collected at beamline ID26 of ESRF and the time-resolved XAFS data was measured at the super XAS beamline of the Swiss Light Source.
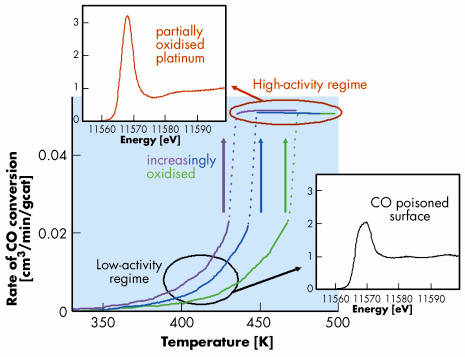
Similar to results on single crystals [2,5], we observed a low- and a high-activity state of the catalyst (Figure 15). These two regimes were only observed at ratios of oxygen to carbon monoxide greater than stoichiometric. A sudden increase in activity to the high-activity regime was observed during heating. This so-called ignition occurred at lower temperature with increasing oxygen concentration. The HERFD spectra, which were obtained every two minutes, showed large changes between spectra taken below and above the ignition temperature. Below ignition, a whiteline of low intensity with a double feature was observed, which is characteristic of platinum particles with adsorbed carbon monoxide [4]. At temperatures above the ignition temperature, the spectra showed a strong increase in the intensity of the whiteline, while the edge energy shifted to lower energy. These features are characteristic of oxidised platinum [4]. The amount of oxidised platinum varied with conversion; at higher conversions, higher amounts of oxide were observed. This suggests that the high-activity regime is characterised by the presence of oxidic platinum, which is most likely present on the surface. During the ignition, the spectra recorded with a time resolution of 0.5 seconds, using quick XANES measurements, showed increasing amounts of platinum oxide, which autocatalytically increased the conversion. This suggests that it is the formation of platinum oxide that enhances the catalytic rate. In contrast to single crystals under low pressures, which are covered by chemisorbed oxygen, nano-sized particles undergo oxidation.
 |
|
Fig. 15: Rate of oxidation of carbon monoxide over 2 wt% Pt/Al2O3 during heating (5 K/min) at oxygen to carbon monoxide ratios of 1 (green), 2 (blue), and 5 (pink). Pt L3 edge HERFD XANES are shown in the low- and the high-activity regime. |
This work showed that the catalyst is in a different structure in the low- and the high-activity regime: adsorbed carbon monoxide on platinum in the low-activity regime, that poisons the surface, and partially oxidic platinum in the high-activity regime as observed with in situ HERFD XAS and time-resolved XAFS. The rate-limiting step in the low-activity regime is desorption of carbon monoxide from the surface and subsequent dissociative adsorption of oxygen. In the high-activity regime, the catalyst surface is oxidised, because of low concentration of carbon monoxide at the catalyst surface. This surface shows a high rate of reaction. High temperature and a high oxygen concentration benefit the formation of the more active catalyst. High resolution XAS enabled the determination of the structure of supported nanoparticles in great detail and of loadings as low as 2 wt%. Because hard X-rays are involved in this experiment, structural information was obtained of the catalyst under real working conditions.
Principal publication and authors
J. Singh (a), E.M.C. Alayon (a), M. Tromp (b), O.V. Safonova (c), P. Glatzel (d), M. Nachtegaal (e), R. Frahm (f), J.A. van Bokhoven (a), Angew. Chem. Int. Ed. 47, 9260 (2008).
(a) Institute of Chemical and Bioengineering, ETH Zürich (Switzerland)
(b) School of Chemistry, University of Southampton (UK)
(c) Swiss Norwegian Beamlines (SNBL), ESRF, Grenoble (France)
(d) ESRF
(e) Paul Scherrer Institute, PSI, Villigen (Switzerland)
(f) Fachbereich C / Physik, University of Wuppertal (Germany)
References
[1] G. Ertl, P.R. Norton, J. Ruestig, Phys. Rev. Lett. 49, 177 (1982); G. Ertl, Surf. Sci., 287-288, 1 (1993).
[2] M.D. Ackermann et al., Phys. Rev. Lett. 95, 255505 (2005).
[3] P. Glatzel, U. Bergmann, Coord. Chem. Rev. 249, 65 (2005).
[4] O.V. Safonova, M. Tromp, J.A. van Bokhoven, F.M.F. de Groot, J. Evans, P.Glatzel, J. Phys. Chem. B 110, 16162 (2006).
[5] X. Su, P.S. Cremer, Y.R. Shen, G.A. Somorjai, J. Am. Chem. Soc. 119, 3994 (1997).



