- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- X-ray Imaging and Optics
- Probing the redox state of iron in silicate melt/glass
Probing the redox state of iron in silicate melt/glass
Iron buffers the redox state of magmas during their ascent from the Earth’s mantle to the surface as it is the most concentrated element having variable oxidation states over the range of natural oxygen fugacities. Assessing the ([Fe3+/(Fe3++Fe2+)] ratio of water-rich melts and those of magmas prior to eruption remains an experimental challenge.
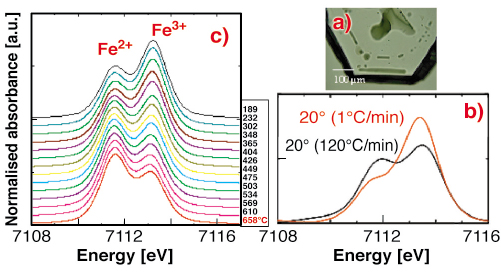
Here we address this problem by in situ measurements of the chemical environment of Fe in natural melt/glass inclusions using micro X-ray absorption near edge structure spectroscopy (XANES) at the Fe-edge. The pre-edge features of Fe2+ and Fe3+ are located on the tail of the main absorption edge in XANES spectra, and they may be discriminated in natural or experimental glasses. Melt/glass inclusions trapped in natural volcanic crystals are suitable experimental systems that give us a snapshot of the magmatic conditions at their time of entrapment, prior to eruption. The melt inclusions studied here were trapped at high temperature (750°C) and preserved as glassy inclusions in natural quartz crystals (Figure 139a) which act as a pressure cell avoiding outgassing during heating. They represent the typical low viscosity peralkaline rhyolitic magma, rich in both H2O (2.5 wt%) and FeO (7 wt%), producing the so-called pantellerite lava, of which Pantelleria island is the type locality.
 |
|
Fig. 139: Quartz-hosted melt inclusions from Pantelleria island a); µXANES spectra collected on ID21 b) and ID24 c) showing the influence of the cooling rate on the pre-edge features. |
Various series of in situ µXANES spectra at the iron K-edge were collected between 800 and 20°C on melt inclusions, using the X-ray microscopy beamline ID21. The quartz hosting the glass inclusions were prepared as double-face polished, 150-190 µm thick lamella in order to preserve the inclusion nearly in the centre of the mineral. They were heated in a water-cooled furnace. The XANES spectra were also acquired for reference glasses, and all were in transmission mode. The energy scan was performed in the so-called continuous mode for which the silicon (220) double crystal monochromator scanned the energy on-the-fly (non stop between two energy points) and the undulator gap and focal distance of the lens were both tuned accordingly. This dead-time free operation mode allows quick energy scans (30 s) with optimum scan reproducibility. The synchrotron X-ray source was demagnified in order to obtain a spot of 2 x 2 µm2.
The main points that arise from the µXANES experiments are: 1) the position of the Fe pre-edge centroid of the 1s ![]() 3d transition correlates with the Fe3+/
3d transition correlates with the Fe3+/![]() Fe ratios in reference glasses; 2) 4Fe3+ prevails in highly oxidised peralkaline glasses, using alkalis for charge balance; 3) the ferrous iron predominates in the melt inclusions at high temperature whereas the intensity of the Fe3+ peak increases upon cooling. Iterative µXANES experiments performed on the same samples show that the process is reversible. The slower the cooling rate, the higher the ferric iron contribution (Figure 139b).
Fe ratios in reference glasses; 2) 4Fe3+ prevails in highly oxidised peralkaline glasses, using alkalis for charge balance; 3) the ferrous iron predominates in the melt inclusions at high temperature whereas the intensity of the Fe3+ peak increases upon cooling. Iterative µXANES experiments performed on the same samples show that the process is reversible. The slower the cooling rate, the higher the ferric iron contribution (Figure 139b).
We also carried out kinetic µXANES experiments on the same series of quartz crystals and inclusions, in dispersion mode, on the XAS beamline ID24 [1]. The µXANES spectra were collected over a range of temperature from 740 to 20°C, with cooling rates varying from ~ 130 to 10 °C/min. Fast acquisition times (7.9 s per spectra) allowed real time monitoring of the chemical environment of iron. These experiments evidence similar variations in the relative intensity of Fe2+ and Fe3+ pre-edges with temperature within a short time span (Figure 139c), involving rather fast kinetics [2].
 |
|
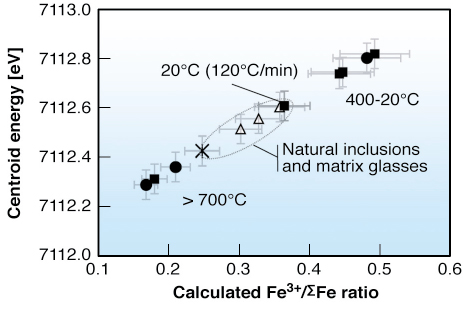
Fig. 140: Variation of the calculated Fe3+/ |
Upon cooling to 20°C, the contrasting behaviour of Fe2+ and Fe3+ pre-edge is accompanied by a variation in the site geometry, with a predominance of 4Fe3+ at low temperature, compared to high temperature melts. It results in an anomalous apparent redox state of iron (Figure 140), when determined against glass standards, rapidly quenched, and where Fe is thus in a different coordination. This apparent oxidation of iron upon cooling is an artefact of changes in Fe coordination. It implies that the [Fe3+/![]() Fe] ratio of glassy samples, measured at 20°C, may be significantly overestimated. This ratio is difficult to retrieve by probing naturally cooled glass inclusions, and most silicate glasses, unless the cooling rate is known. We show here that high temperature µXANES experiments led to an assessment of the ferric-ferrous ratio in the water-rich peralkaline melt in pre-eruptive magmatic conditions.
Fe] ratio of glassy samples, measured at 20°C, may be significantly overestimated. This ratio is difficult to retrieve by probing naturally cooled glass inclusions, and most silicate glasses, unless the cooling rate is known. We show here that high temperature µXANES experiments led to an assessment of the ferric-ferrous ratio in the water-rich peralkaline melt in pre-eruptive magmatic conditions.
References
[1] S. Pascarelli, O. Mathon and G. Aquilanti, J. of Alloys and Compounds 362, 33 (2004).
[2] N. Métrich, J. Susini, M. Munoz, S. Pascarelli and D. Massare, Geophysical Research Abstract 7, 08840 (2005).
Principal Publication and Authors
N. Métrich (a), J. Susini (b), E. Foy (a), D. Massare (a), F. Farges (c), S. Lequien (a), L. Sylla (a) and M. Bonnin-Mosbah (d), Chemical Geology 231, 350-363 (2006).
(a) Laboratoire Pierre Süe, CNRS-CEA, CE-Saclay, Gif sur Yvette (France)
(b) ESRF
(c) Laboratoire de Geosciences, Université de Marne La Vallée (France)
(d) INSTN, CEA, CE-Saclay (France)



