- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- Surface and Interface Science
- In situ electrochemical and SR-XRD time-resolved study of lead carboxylate coating for the protection of cultural heritage artefacts
In situ electrochemical and SR-XRD time-resolved study of lead carboxylate coating for the protection of cultural heritage artefacts
European lead heritage ranges from simple ornaments to vast artistic and engineering masterpieces such as pipe organs (Figure 94) - all in danger of decay and loss through corrosion. Indeed, lead organ pipes are amongst the most seriously jeopardised artefacts: their sound is critically dependent on the material, as well as their shape, size, and condition. Corrosion usually starts at the foot of the pipe and proceeds invisibly from the inside towards its mouth, resulting in changes to the tonal quality due to distortion of the mouth geometry. Cracks and holes then develop, at which point the pipe becomes mute and requires repair or replacement [1]. Lead is seriously affected by the presence of organic acids (formic, acetic...) in the environment, and both original and restored wood (e.g. in the wind chest) are a source of these.
 |
|
Fig. 94: The Stellwagen organ in St Jakobi church in Lübeck. The prospect pipes dating from 1467 have suffered heavy corrosion on the inside (Photo courtesy C.J. Bergsten). |
Coating lead artefacts to protect them is fraught with difficulty since badly researched coatings and treatments can lead to irreversible changes in appearance and function, and even more rapid degradation. Only after meticulous investigation can a coating be applied with relative confidence. An attractive feature of the carboxylate coating process is simplicity. The lead is soaked in a non-toxic solution of sodium decanoate [2]. The result is a dark coating, similar to aged lead in appearance. In this initial study, we set out to measure the growth characteristics of such layers using time resolved X-ray diffraction (XRD), electrochemical impedance spectroscopy (EIS), scanning electron microscopy (SEM) and mass gain measurements, as a prelude to testing its performance in a corrosive environment.
On BM28 (XMaS) we made simultaneous EIS and X-ray diffraction (XRD) measurements in a novel electrochemical cell (the eCell), developed especially for the characterisation of rough heterogeneous metal surfaces and the characterisation of conservation methods using synchrotron-based techniques. A particular objective is to obtain correlated, time-resolved spectroelectrochemical data. eCell [3] is an adaptive kit consisting of the cell itself, a range of mounting options, a custom miniature potentiostat, and a data system running on a notebook PC.
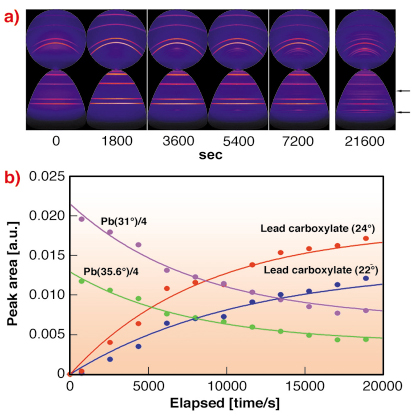
An especially important feature of XRD is the use of a fast 2D detector such as the Mar CCD 165 to collect the data. The sample cannot easily be rotated to randomise the diffraction pattern because it is the working electrode in an electrochemical cell whose operation would be disturbed by the motion. However, the samples are polycrystalline and may contain large crystals or some preferred orientation so that a 1D detector can give misleading and apparently irreproducible spectra. An image can be integrated to provide more random spectra. Intensity variations in the rings can indicate differences or similarities in grain size and orientation of the metal substrate and its coating. The geometry of the experiments dictates that the camera axis is aligned between 20 and 50 degrees to the incoming beam, so that the intersections between the diffraction cones and the camera plane are elliptical. Usually the diffraction centre is well below the camera plane and the rings are incomplete. Therefore, we have developed a package, esaProject, which centres the images and then transforms them into a space where the rings are linear (Figure 95). Thus, spectra corrected for the arc length from all or part of the image can then be extracted and processed further.
 |
|
Fig. 95: a) XRD images (upper row) measured in situ and esaProject transforms (lower row) from the developing coating. The lead carboxylate regions are arrowed, other rings are due to lead. b) Areas of the X-ray peaks from the images as a function of time. |
Figure 95a shows a time sequence of images and their reprojection from a growing lead carboxylate coating. In detail, the lead rings are spotty whereas the carboxylate rings are smooth, which is indicative of a dense interlocking microcrystalline structure, ideal in a potential protective coating. Figure 95b shows the change in peak area extracted from a sequence of CCD images. That the lead is covered over is clearly evident and the data correlate well with the mass gain measurements. The simultaneous EIS measurements are indicative of the development of a high integrity layer free from pinholes.
The combination of XRD and EIS shows that the carboxylate coating is promising and has the basic characteristics required for a protective coating on lead. Future work will establish its durability.
References
[1] For more information see the website of the FP5 project COLLAPSE at www.goart.gu.se/collapse/.
[2] E. Rocca, C. Rapin and F. Mirambet, Corrosion Science 46, 653-665 (2004).
[3] M. G. Dowsett and A. Adriaens, Analytical Chemistry 78, 3360-3365 (2006).
Principal Publication and Authors
M.G. Dowsett (a), A. Adriaens (b), B. Schotte (b), G.K.C. Jones (a) and L. Bouchenoire (c), manuscript in preparation.
(a) Departments of Physics, University of Warwick, Coventry (UK)
(b) Department of Analytical Chemistry, Ghent University (Belgium)
(c) ESRF



