- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- Surface and Interface Science
- Out-of-plane Distortion of a Molecule at the Interface with a Metal
Out-of-plane Distortion of a Molecule at the Interface with a Metal
When a molecule adsorbs at a surface, an obvious questions to ask is whether the internal structure of the adsorbate is changed by the interaction with the substrate. This has been studied in detail for small molecules such as CO. Yet, for the interfaces of large planar aromates with metals, which have recently become a focus of considerable research activity, very little data on the internal structure of the adsorbate is available. Even the adsorption height, i.e. the length of the molecule-substrate bond, has only been measured in very few cases [1]. This sparsity of experimental data contrasts with the importance of ![]() -conjugated adsorbates in the fields of organic and molecular electronics, where characterisation and control of contacts between molecules and metal electrodes pose major challenges for further development.
-conjugated adsorbates in the fields of organic and molecular electronics, where characterisation and control of contacts between molecules and metal electrodes pose major challenges for further development.
 |
|
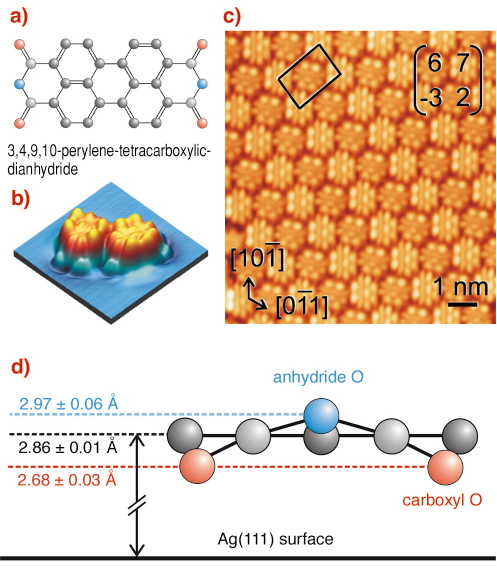
Fig. 103: (a) Chemical structure of PTCDA. (b) STM image of two individual PTCDA molecules on Ag(111). The imaged local density of states resembles the LUMO of the free molecule. (c) STM image of the PTCDA/Ag(111) interface, with superstructure unit cell and matrix, and crystallographic directions of Ag(111). (d) Side view of the molecule, showing the vertical adsorption geometry. The vertical length scale is expanded by a factor 3. |
We have carried out normal incidence X-ray standing wave (NIXSW) experiments on the PTCDA/Ag(111) interface at beamline ID32, making use of the UHV chamber facility. Perylene-tetracarboxylic-dianhydride (PTCDA), Figure 103a-b, is a prototypical organic semiconductor. It forms highly-ordered structures on many substrates and is therefore well suited for studies concerning fundamental interface properties. If combined with photoelectron detection, X-ray standing wave techniques unite the structural accuracy of X-ray scattering methods with the chemical sensitivity of photoemission and thus allow the element-specific determination of adsorption heights. For planar molecules containing different atomic species, the technique thus uniquely permits the precise measurement of vertical adsorbate distortions. The well-defined site-specific interaction between PTCDA and Ag(111) (Figure 103c) makes this system ideally suited for an exemplary study of substrate-induced distortions in a complex planar adsorbate.
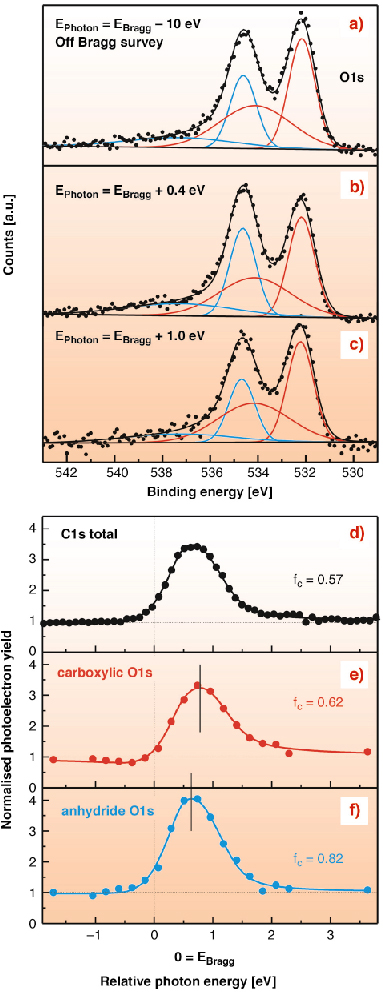
The PTCDA molecule consists of a perylene core with two dianhydride groups. Since the O1s signals of the carboxylic and the anhydride oxygen are chemically shifted by ~ 2.4 eV, the position of three distinct species can be determined (Figure 103d). We find an average carbon-silver distance of 2.86 Å. Compared to the interlayer distance in bulk PTCDA (3.22 Å), this is a rather short bond length, indicating a sizable chemical interaction. Moreover, we observe a distortion of the molecule in which carboxylic oxygens bend down, while the anhydride oxygens move up. In fact, the height difference between the two oxygen species can already be inferred qualitatively from the observation that the two corresponding O1s peaks reach their respective maxima at slightly different X-ray energies (Figure 104a-c). Quantitative analysis of the photoelectron yield curves in Figure 104e-f yields positions of 2.68 Å and 2.97 Å for Ocarboxylic and Oanhydride, respectively.
 |
|
Fig. 104: (a)-(c) O1s spectra recorded at various X-ray energies close to the (111)-Bragg-energy of 2623.2 eV. (d)-(f) Photoelectron yield curves fitted in dynamic X-ray scattering theory, with corresponding coherent fractions. |
The observed distortion raises questions concerning the bonding mechanism of PTCDA on Ag(111). From electron spectroscopies and the submolecular scanning tunneling contrast shown in Figure 103b-c, we know that the bonding is chemisorptive, and that it occurs mainly on the perylene core of PTCDA. However, it was also clear that the anhydride groups must be important, too, since the bonding of perylene on Ag(111) is considerably weaker [2]. What, then, is the role played by the downward-bent carboxylic oxygen atoms? Does the buckling indicate a bonding via the carboxylic oxygens?
Analysis of the bonding confirms the dominating role of the perylene core, but also indicates the formation of weak Ag-O bonds involving carboxylic oxygen atoms. This can be understood in analogy to the established theory of CO chemisorption: For PTCDA as for CO, carbon-derived frontier orbitals participate in a strong charge exchange with the metal, and as a result, certain intramolecular bonds are weakened. In the case of PTCDA/Ag(111) this weakening of intramolecular bonds materialises as a lowering and secondary bonding of the carboxylic oxygen to the Ag(111) surface.
In summary, our NIXSW experiments, together with the lateral structure of PTCDA/Ag(111) known from STM and LEED, yield one of the first comprehensive structural models of a complex adsorbate on a metal surface, comprising the adsorption site as well as internal adsorbate distortions. We are currently extending this work to different bonding situations and substrates, with the aim to generate a more complete structural database for the bonding of this model molecule to transition metal surfaces.
References
[1] L. Kilian et al. Phys. Rev. B 66, 075412/1-9 (2002).
[2] M. Eremtchenko et al., Nature 425, 602 (2003).
Principal Publication and Authors
A. Hauschild (a), K. Karki (b), B.C.C. Cowie (c), M. Rohlfing (b), F.S. Tautz (b), and M. Sokolowski (a), Phys. Rev. Lett. 94, 036106 (2005) and Phys. Rev. Lett. 95, 209602 (2005).
(a) Institut für Physikalische und Theoretische Chemie der Universität Bonn (Germany)
(b) School of Engineering and Science, International University Bremen (Germany)
(c) ESRF



