- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- Soft Condensed Matter
- X-ray Diffraction Reveals a New Mechanism of Muscle Contraction
X-ray Diffraction Reveals a New Mechanism of Muscle Contraction
Muscle force results from the interaction of the globular heads of myosin molecules with actin filaments. The structure-function relationship in the myosin motor was studied with low-angle time-resolved X-ray diffraction in contracting muscle fibres where force generation was triggered by temperature jump or length step. Both types of perturbations induce simultaneous changes in the muscle force and in the extent of labelling of the actin helix by stereo-specifically bound [1] myosin heads at a constant total number of attached heads [2]. The generally accepted hypothesis [3] assumes that muscle force is generated solely by tilting of the light chain domain of the myosin head about its catalytic domain, or by the lever arm mechanism. Data obtained suggest an additional force-generating step: the ‘roll and lock' transition of catalytic domains of non-stereo-specifically attached heads to a stereo-specifically bound state. A suggested model quantitatively explains the data.
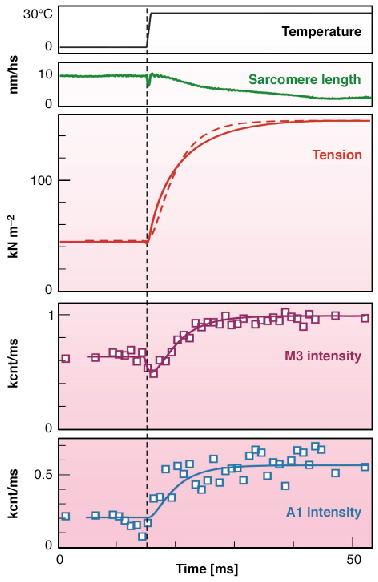
The temperature jump from 5°C to 30°C in permeabilised fibres from rabbit muscle induced a three-fold increase in active tension. Time course of changes in tension and in the 2D X-ray diffraction pattern collected at ESRF ID02 with the RAPID detector (Daresbury Laboratory, UK) from muscle fibres with 1 ms time resolution is shown in Figure 66. It was found that the intensities of all recorded layer lines originating from the actin helix increase with temperature in parallel with tension. When myosin heads bind actin stereo-specifically they adopt the symmetry of the actin helix so that the axial spacing of the layer lines remains the same but the intensities are higher as they are determined by the number of heads incorporated in the actin helix.
 |
|
Fig. 66: Time course of mechanical and structural responses to the temperature jumps. Top to bottom: averaged temperature, sarcomere length, averaged tension, IM3, and IA1. The smooth lines on IM3 and IA1 data and the dashed line on the tension trace are the results of modelling. The vertical line marks the time when the 1ms–long T-jump was half-complete. |
The apparent rate constants of the rise in tension and in IA1 (intensity of the first actin layer line) were about 200 s-1. The intensity of the third myosin reflection, IM3, dropped during the temperature jump and then increased with an apparent rate constant similar to that for tension. Temperature independence of fibre stiffness [2] indicates that the number of myosin heads attached to actin does not change with temperature and that the increase in tension with temperature is caused by an increase in force produced by each head. The significant increase in IA1 after the temperature jumps must be caused by a stereo-specific 'locking' of myosin heads on actin. The contribution of non-stereo-specifically bound heads to the layer line intensities is low because the distribution of their electron density in space does not follow helical symmetry.
 |
|
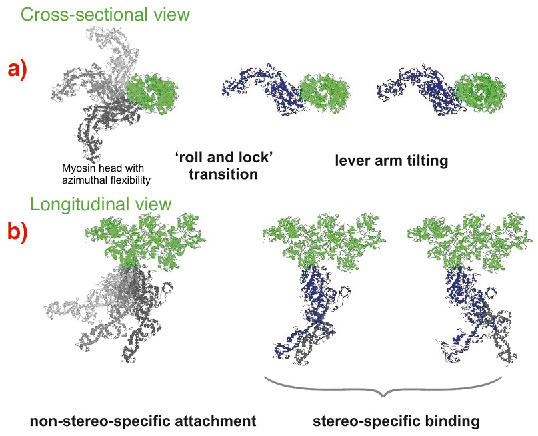
Fig. 67: Suggested scheme of the mechanism of force generation by myosin heads. Attached states of a myosin head (blue or grey) bound to actin (green) are shown in two projections with the actin axis: (a) azimuthal and (b) axial. Force or displacement is produced during both the 'roll-and-lock' transition and the lever-arm tilting. |
A kinetic and structural model (Figure 67) was suggested. The structure-function relations obtained from time-resolved mechanical and structural data as well as the known kinetic data were integrated to link together the lever arm mechanism [3] with the idea of a preceding stereo-specific 'locking' of the myosin molecule on the actin filament. The 'roll and lock' mechanism suggested here and the kinetic and structural model based on this mechanism quantitatively explain both the mechanical and the structural behaviour of myosin heads in temperature jump experiments and specifies structural and kinetic characteristics of the two-step force generation by muscle myosin. An increase in IA1 after muscle shortening predicted by the 'roll and lock' model was verified experimentally. The two-step force generation mechanism suggested here is consistent with the electron microscope tomography on insect flight muscles [4] where myosin heads in configurations similar to those assumed for attached states of our model were observed.
References
[1] S. Bershitsky, A. Tsaturyan, O. Bershitskaya, G. Mashanov, P. Brown, R. Burns and M. Ferenczi, Nature 388, 186 (1997).
[2] S. Bershitsky and A. Tsaturyan, J. Physiol. 540, 971 (2002).
[3] K. Holmes, Curr. Biol. 7, R112 (1997).
[4] K. Taylor, H. Schmitz, M. Reedy, Y. Goldman, C. Franzini-Armstrong, H. Sasaki, R. Tregear, C. Lucaveche, R. Edwards, L. Chen, H. Winkler and M. Reedy, Cell 99, 421 (1999).
Principal Publication and Authors
M. Ferenczi (a), S. Bershitsky (b), N. Koubassova (c), V. Siththanandan (a), W. Helsby (d), P. Panine (e), M. Roessle (e), T. Narayanan (e) and A. Tsaturyan (c), Structure 13, 131 (2005).
(a) Imperial College London (UK)
(b) Institute of Immunology and Physiology, Yekaterinburg (Russia)
(c) Institute of Mechanics, Moscow University, Moscow (Russia)
(d) Daresbury Laboratory, Cheshire (UK)
(e) ESRF



