- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- High Resolution and Resonance Scattering
- Photosynthetic Dioxygen Formation Tracked by X-ray Experiments with Microsecond Resolution
Photosynthetic Dioxygen Formation Tracked by X-ray Experiments with Microsecond Resolution
In plants, algae and cyanobacteria, electrons and protons are extracted from water molecules in a light-driven process denoted as photosynthetic water oxidation. Thereby the atmospheric dioxygen (O2) is formed. The reactions are catalysed by a tetra-manganese complex bound to the proteins of photosystem II (PSII). For more than 30 years, the paradigm for understanding O2 evolution has been the S-state cycle model [1] of water oxidation involving transitions between five states (S0, S1,…S4) of the Mn complex. Dioxygen is formed in the S3 ![]() S0 transition. A crucial intermediate of this transition, the S4-state, remained enigmatic.
S0 transition. A crucial intermediate of this transition, the S4-state, remained enigmatic.
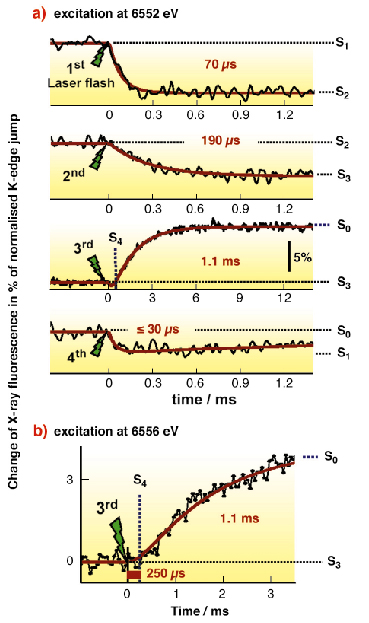
One round of the S-cycle requires four light quanta. Here, these were provided by successive laser flashes that drive isolated PSII particles through the reaction cycle. Each light flash leads to the formation of an oxidised tyrosine residue (TyrZ•) which then extracts an electron from the Mn complex. We monitored the redox processes of the protein-bound Mn complex by fluorescence-detected X-ray absorption spectroscopy (XAS) at the Mn K-edge in real time [2]. Facilitated by the high flux and stability of the X-ray beam at beamline ID26, for the first time we could follow changes in the Mn X-ray fluorescence after laser-flash illumination of PSII samples with a time resolution of 10 µs thereby directly watching the S-transitions (Figure 22a). At an excitation energy of 6552 eV, for S1 ![]() S2 (first flash), S2
S2 (first flash), S2 ![]() S3 (second flash), and S0
S3 (second flash), and S0 ![]() S1 (fourth flash), the exponential absorption decrease indicated oxidation of the Mn complex with the halftimes shown in Figure 22.
S1 (fourth flash), the exponential absorption decrease indicated oxidation of the Mn complex with the halftimes shown in Figure 22.
 |
|
Fig. 22: (a) Oxidation and reduction of the Mn complex of PSII monitored by time-resolved X-ray measurements. Nanosecond flashes (20 ms duration) of green Laser light initiated the S-state transitions in PSII samples monitored by X-ray fluorescence (excitation energy of 6552 eV). The time course of the Mn K |
The third Laser flash induced the S3 to S0 transition. An absorption increase due to Mn reduction by the substrate water upon O2 formation was observed. The absorption increase was preceded by a lag phase of about 250 µs duration (Figure 23). This lag phase suggested a kinetically resolvable intermediate. However, a minor fraction of PSII that undergoes the S2 ![]() S3 transition on the third flash (due to PSII which did not turn over on the first laser-flash) might mimic a delay phase in the S3
S3 transition on the third flash (due to PSII which did not turn over on the first laser-flash) might mimic a delay phase in the S3 ![]() S0 transition. A time-resolved XAS experiment at 6556 eV clarified the situation since no change in the X-ray absorption is observed for S2
S0 transition. A time-resolved XAS experiment at 6556 eV clarified the situation since no change in the X-ray absorption is observed for S2 ![]() S3 at this energy. A sizable lag phase still was present (Figure 22b), proving the existence of a kinetically resolvable intermediate. The intermediate is formed prior to the Mn-reducing/O2-forming step and thus represents the long-searched-for S4-state.
S3 at this energy. A sizable lag phase still was present (Figure 22b), proving the existence of a kinetically resolvable intermediate. The intermediate is formed prior to the Mn-reducing/O2-forming step and thus represents the long-searched-for S4-state.
 |
|
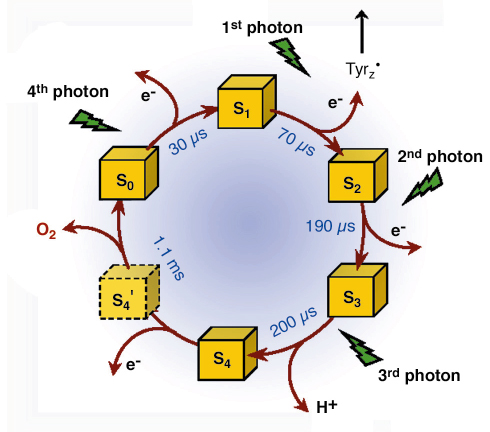
Fig. 23: Extension of the classical S-state cycle of photosynthetic oxygen evolution. The half-times of the S-transitions were determined by the X-ray experiment at the Mn K-edge. Existence and formation rate of the S4 state have been uncovered in the present investigation. S4 is not formed by electron transfer to TyrZ•, but by a deprotonation reaction and represents the starting point for O-O bond formation. S4’ denotes a hypothetical intermediate where four electrons have been extracted from the Mn complex, but O2 has not been formed. |
Further characterisation of the intermediate enabled us to identify the chemical nature of the S4-state. We propose a ‘proton-first’ reaction sequence on the oxygen-evolving transition, where the TyrZ• radical induces, likely electrostatically, the deprotonation reaction which is a prerequisite to the subsequent electron transfer from the manganese/water complex to TyrZ•. Our identification of the S4 state will spur further investigations on this key step in photosynthetic water oxidation. It leads to an extension of the classical S-state cycle because the deprotonation must be followed by electron transfer to TyrZ•, this implying an S4’-state (Figure 23). The described progress was facilitated by a novel time-resolved X-ray experiment. This represents another step forward towards a structural biologist’s dream: watching biological function in real time.
Acknowledgements
We thank Drs. T. Neisius, S. Eeckhout, and P. Glatzel (all ESRF) for excellent support and P. Loja (our group) for her important contributions to XAS data collection. Financial support by the Deutsche Forschungsgemeinschaft (SFB 498, projects C6 and C8) and the Bundesministerium für Bildung und Forschung (grant 05KS1KEA/6) is gratefully acknowledged.
References
[1] B. Kok, B. Forbush, M. McGloin, Photochem. Photobiol. 11, 457-475 (1970).
[2] M. Haumann, C. Müller, P. Liebisch, L. Iuzzolino, J. Dittmer, M. Grabolle, T. Neisius, W. Meyer-Klaucke, H. Dau, Biochemistry 44, 1894-1908 (2005).
Principal Publication and Authors
M. Haumann, P. Liebisch, C. Müller, M. Barra, M. Grabolle, H. Dau, Science 310, 1019-1021 (2005).
Freie Universität Berlin, FB Physik (Germany)



