- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2002
- Surface and Interface Science
- Compressibility of CO Adsorbed on Ni(111) from 10-6 mbar to 1.2 bar Ambient CO Pressures Investigated with X-ray Diffraction
Compressibility of CO Adsorbed on Ni(111) from 10-6 mbar to 1.2 bar Ambient CO Pressures Investigated with X-ray Diffraction
Gas molecules adsorbed on solid surfaces have been one of the main topics in surface science in the last two decades. From a fundamental point of view, they make an excellent test case for the investigation of two-dimensional thermodynamic processes such as order-disorder phenomena. From a more practical side, their study is a pre-requisite for the understanding of the microscopic mechanisms underlying heterogeneous catalysis. Most of the experimental work to date was done using electron-based techniques with the surface of the samples in high or ultra high vacuum (UHV) environments so that structural studies on chemisorbed layers could be performed while avoiding attenuation of the electron beams by the gasses. This limitation excluded the possibility of investigating adsorbed layers in equilibrium with their vapour under conditions like those encountered in common industrial reactions where significant adsorption and desorption rates exist. Fortunately, X-rays are not limited by such strong attenuation in the gas phase and so they can be used in crystallographic studies of chemisorbed layers at atmospheric pressures.
We have studied the adsorption of CO at room temperature under equilibrium conditions in the pressure range 10-6 mbar - 1.2 bar. The experiments were performed at ID3, the Surface Diffraction Beamline, with X-rays of wavelength 0.74 Å and a specially-designed UHV/high pressure chamber (base pressure 1 x 10-9 mbar) mounted on a high-precision diffractometer.
 |
|
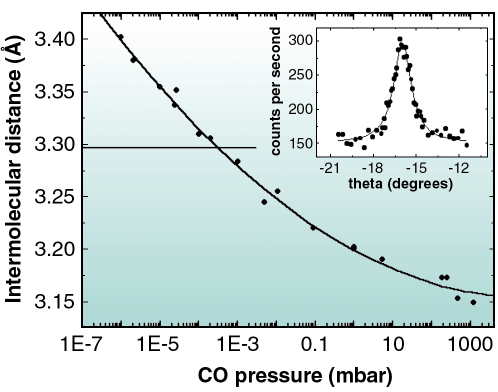
Fig. 39: Inset: rocking scan of a diffraction peak from the adsorbed CO layer taken at p = 1.2 bar in the gas phase. Main graph: dependence of the intermolecular distance of adsorbed CO on Ni(111) with the CO pressure in the chamber. The horizontal line marks the nearest neighbour distance for the ( |
At p = 10-3 mbar, the commensurate (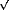 7x
7x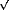 7)R19.1° structure [1] was observed. Figure 39 shows that below this pressure, an expansion of the unit cell occurred (and conversely above this pressure a contraction occurred), as deduced from the magnitudes of the reciprocal lattice vectors from the adlayer, leading to incommensurate structures. Relative to the
7)R19.1° structure [1] was observed. Figure 39 shows that below this pressure, an expansion of the unit cell occurred (and conversely above this pressure a contraction occurred), as deduced from the magnitudes of the reciprocal lattice vectors from the adlayer, leading to incommensurate structures. Relative to the 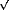 7 periodicity, the experimentally determined lattice parameter increased by 3.2% at 10-6 mbar and decreased by 4.4 % at 1.2 bar. Interestingly, these changes were reversible with the gas pressure. The pressure-induced contraction or expansion of the overlayer results in incommensurate structures with the Ni substrate, as it is the case for the technologically relevant pressure of one atmosphere. This result might be of importance for fundamental studies of catalysis.
7 periodicity, the experimentally determined lattice parameter increased by 3.2% at 10-6 mbar and decreased by 4.4 % at 1.2 bar. Interestingly, these changes were reversible with the gas pressure. The pressure-induced contraction or expansion of the overlayer results in incommensurate structures with the Ni substrate, as it is the case for the technologically relevant pressure of one atmosphere. This result might be of importance for fundamental studies of catalysis.
The isothermal compressibility of a 2D system in equilibrium with an ideal 3D gas at a pressure P is given by K = -(kT)-1 ( a/
a/ lnP)T where k is the Boltzmann constant and a is the molecular area (a =
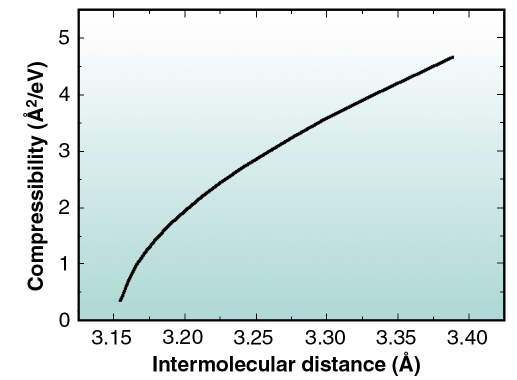
lnP)T where k is the Boltzmann constant and a is the molecular area (a = 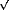 3/2 D2). By applying the above definition to the continuous curve in Figure 39, the dependence of K with D may be obtained as displayed in Figure 40. As it may be seen, at atmospheric pressure the compressibility is about 0.3 Å2/eV, which is about 15 times larger than at low pressures. The above value, which may be compared to that of Pb electrodeposited on Ag(111) (1 Å2/eV [2]) and to that of Ar on graphite at 42 K (35 Å2 /eV [3]), indicates that the CO layer is very stiff at the pressures relevant for catalytic reactions. To our knowledge, the results in Figures 39 and 40 are the only published data of 2D compressibility of a reactive gas over a large pressure range that includes the technologically important pressure of one atmosphere.
3/2 D2). By applying the above definition to the continuous curve in Figure 39, the dependence of K with D may be obtained as displayed in Figure 40. As it may be seen, at atmospheric pressure the compressibility is about 0.3 Å2/eV, which is about 15 times larger than at low pressures. The above value, which may be compared to that of Pb electrodeposited on Ag(111) (1 Å2/eV [2]) and to that of Ar on graphite at 42 K (35 Å2 /eV [3]), indicates that the CO layer is very stiff at the pressures relevant for catalytic reactions. To our knowledge, the results in Figures 39 and 40 are the only published data of 2D compressibility of a reactive gas over a large pressure range that includes the technologically important pressure of one atmosphere.
 |
|
Fig. 40: Compressibility of the adsorbed CO as a function of the nearest neighbour intermolecular distance. |
In conclusion, we have observed the reversible compression/expansion of adsorbed CO on Ni(111) at room temperature by means of surface X-ray diffraction. The compressibility of the adsorbed layer has been obtained over 9 decades in pressure up to the atmosphere. At pressure values relevant for realistic catalytic conditions, most of the CO molecules sit in low symmetry sites and the layer is stiff.
References
[1] G. Held, J. Schuler, W. Skalarek and H.P. Steinruck, Surf. Sci., 398, 154-171 (1998).
[2] O. R. Melroy, M. F. Toney, G. L. Borges, M. G. Samant, J. B. Kortright, P. N. Ross and L. Blum, Phys. Rev. B, 38, 10962-10965 (1988).
[3] C. G. Shaw and S. C. Fain Jr., Surf. Sci. 83, 1-10 (1979).
Principal Publication and Authors
C. Quirós (a), O. Robach (a), H. Isérn (a), P. Ordejón (b) and S. Ferrer (a), Surf. Sci. 522, 161 (2003).
(a) ESRF
(b) Institut de Ciencia de Materials de Barcelona, CSIC, Bellaterra (Spain)



