- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Life Sciences
- Crystal Structure of T Cell Receptors Bound to an Allogeneic MHC Molecule
Crystal Structure of T Cell Receptors Bound to an Allogeneic MHC Molecule
The T cells play a central role in vertebrate immune systems by protecting the organism against the proliferation of different infectious agents such as viruses, bacteria or protozoa and of some kinds of cancerous cells. They mediate the cellular immune response whose role is to detect and eliminate foreign agents or their products inside the host cells. However, their strong reaction to the presence of foreign cells also makes them the cause of graft rejection and graft-vs.-host diseases.
T cells possess specific receptors on their surface, the ß T cell receptors (TCRs), that are able to discriminate between peptide fragments derived from self or foreign proteins, which are presented by specific proteins, the major histocompatibility complex (MHC) molecules. However, the specificity of the TCR is functionally degenerate: one TCR is able to recognise numerous peptides and several MHC molecules. The TCR cross-reactivity for several peptides is essential to insure that at least one T cell clone, among the large but finite range, reacts with any one of the millions of putative foreign peptides. Graft rejection is governed by the productive binding of a TCR with an intraspecies allelic variant of a self-MHC molecule (i.e. an MHC molecule from another individual, also named allogeneic MHC) this is termed allo-reactivity.
The understanding of the structural basis of TCR specificity for both the peptide and the MHC molecule should provide us with precious information for the elaboration of new protocols either to specifically activate the immune system (against retro-viral infection, cancer) or in contrast to increase its tolerance (to prevent graft rejection).
The first structures of TCR/peptide/MHC complexes have established the general lines along which the TCR interacts with the composite surface made of both the self-MHC molecule and foreign peptide amino-acids. However, the way a TCR was able to recognise an allogeneic MHC molecule had not been determined. Two hypotheses were proposed: 1) TCR directly recognises the amino-acids which differ between self and allogeneic MHC molecules; and 2) TCR recognises as foreign the different set of peptides that are presented by allogeneic MHC molecules.
In order to clarify this point, we have solved the structure of two murine TCRs (BM3.3 and KB5-C20) in complex with an allogeneic MHC molecule (H-2Kb) loaded with a self-peptide. The first structure has been determined last year [1] and the second one very recently, thanks to crystallographic data collected at the ESRF beamline BM30A [2].
These two structures provide us with pioneering results on the structural basis of TCR allo-recognition (Figure 16). They clearly support the second assumption i.e. that TCR recognises as foreign the different set of peptides that are presented by allogeneic MHC molecules. Our results shows that allo-recognition requires self and allogeneic MHC molecules to have nearly identical TCR-facing residues, implying that graft rejection and graft-vs.-host diseases are essentially mediated by the different peptide repertoires presented by allogeneic MHC molecules. Consequently and quite surprisingly, an allogeneic MHC molecule that significantly differs in its TCR facing residues may not elicit T cell activation. Thus, when selecting graft donors, it may be useful to look not only for MHC molecules identical to those of the graft host, but also for those displaying significant dissimilarities.
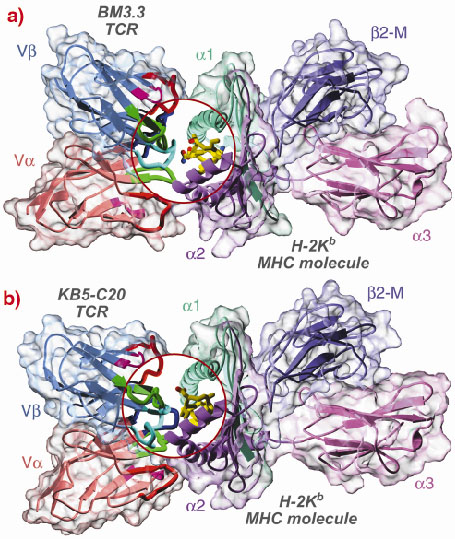 |
Fig. 16: Backbone representation of the BM3.3 TCR/pBM1/H-2Kb MHC (a) and KB5-C20/pKB1/H-2Kb MHC (b) ternary complexes. The TCR variable domains are composed of 2 Ig-like domains: V |
References
[1] J.-B. Reiser, C. Darnault, A. Guimezanes, C. Grégoire, T. Mosser, A.-M. Schmitt-Verhultz, J.C. Fontecilla-Camps, B. Malissen, D. Housset and G. Mazza, Nature Immunol., 1, 291-297 (2000).
[2] J.-B. Reiser, C. Grégoire, C. Darnault, T. Mosser, A. Guimezanes, A.-M. Schmitt-Verhultz, J.C. Fontecilla-Camps, G. Mazza, B. Malissen and D. Housset, Immunity, accepted.
Authors
J.-B. Reiser (a), C. Darnault (a), J.C. Fontecilla-Camps (a), D. Housset (a), G. Mazza (b), T. Mosser (b), C. Grégoire (b), A. Guimezanes (b), A.-M. Schmitt-Verhultz (b) and B. Malissen (b).
(a) LCCP, I.B.S. J.P. Ebel, CEA-CNRS-UJF, Grenoble (France)
(b) C.I.M.L., INSERM-CNRS, Marseille (France)



