- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Life Sciences
- Bacterial Origin of the Actin Cytoskeleton
Bacterial Origin of the Actin Cytoskeleton
A defining feature of bacterial cells has long been thought to be the lack of a cytoskeleton, which in eukaryotes is indispensable for cell division, for the maintenance of cell shape, and numerous other functions. The cytoskeleton is composed of actin filaments, microtubules, and intermediate filaments. Microtubules are thick polymers that, apart from other functions, form the mitotic spindle to pull the chromosomes apart during cell division. The actin filaments are relatively thin, and are cross-linked into larger structures to obtain mechanical integrity. They are located just underneath the cell cortex and are involved in determination of the cell shape.
In recent years it became apparent that the microtubule-based cytoskeleton can be traced back to a bacterial protein called FtsZ (filamentous-temperature sensitive protein Z). FtsZ is structurally very similar to tubulin and forms filaments in an analogous manner that mediate bacterial cell division. Despite the link between FtsZ and tubulin, the bacterial homologue of actin remained obscure.
In March 2001, Laura Jones of the group of Jeff Errington (University of Oxford, UK) looked at the cellular distribution of MreB, a bacterial protein, predicted to be a member of the actin family. She showed by immunofluorescence that MreB from Bacillus subtilis forms spiral-like structures underneath the cell membrane [1]. Depletion of MreB from B. subtilis caused a defect in cell shape. The distribution of MreB-like genes among the bacterial subkingdoms shows that bacteria with a non-spherical cell possess one or more MreB-like genes [1]. This strongly suggests that there is a MreB-based cytoskeleton in bacteria that maintains their cell shape.
If MreB would be the true actin homologue, could it self-assemble into filaments?
We have recently shown that purified MreB from Thermotoga maritima can form polymers in vitro under similar conditions to eukaryotic actin [2]. A closer look at these polymers under the electron microscope showed that they consist of pairs of protofilaments, each being a string of monomers. The spacing between the monomers is 51 Å, which is very similar to the 55 Å spacing found for filamentous actin [2]. The similarity between actin and MreB became even more convincing when the crystal structure of T. maritima MreB was solved by MAD (multiple anomalous dispersion), at the ESRF beamline ID14-4. This showed that on the structural level MreB and actin are the most closely related proteins of the actin family. The trigonal MreB crystals (P3121, a = b = 51.58 Å, c = 292.37 Å) diffracted to 2.1 Å, and were partially twinned. A surprising property of the crystals is that the subunits are already assembled into protofilaments. The crystals thus reveal a detailed look at the interface between the subunits in the protofilament. A comparison between a single protofilament of actin and MreB shows that the subunits are in almost identical orientation in the protofilament, resulting in a similar spacing between the monomers (Figure 17). Combining the X-ray and electron microscopic data, we conclude that MreB and actin form the same protofilaments when polymerised and that MreB is the bacterial homologue of actin.
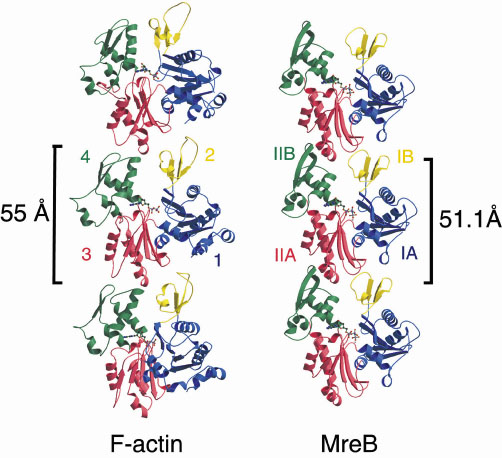 |
Fig. 17: A comparison of a single protofilament of F-actin and MreB. Three subunits are shown from each polymer, each composed of four domains, depicted in blue, yellow, red and green. The longitudinal spacing is similar for F-actin and MreB (55 and 51 Å, respectively). |
There is one striking difference between the MreB polymers and F-actin. In eukaryotic actin two protofilaments gently twist around each other to form a helical F-actin filament, whereas bacterial actin assembles into pairs of straight protofilaments. This may indicate that the propensity of actin to form helical filaments has evolved after the eukaryotic cell developed from its bacterial origins.
References
[1] L.J.F. Jones, R. Carballido-Lopez and J. Errington, Cell, 104, 913-922 (2001).
[2] F. van den Ent, L.A. Amos and J. Löwe, Nature, 413, 39-44 (2001).
Authors
F. van den Ent, L.A. Amos and J. Löwe.
MRC-Laboratory of Molecular Biology, Cambridge (UK)



