- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Chemistry
- Presence of Sulfite (SIV) in Magmas: Implications for Volcanic Sulphur Emissions
Presence of Sulfite (SIV) in Magmas: Implications for Volcanic Sulphur Emissions
Excess degassing of sulphur dioxide (SO2) during eruptions has been detected at numerous volcanoes, particularly in subduction zones. There is a growing consensus that gaseous sulphur-containing species (SO2, H2S) can be transferred into the magmatic vapour phase prior to eruption during either gas accumulation in long-lived shallow reservoirs or continuous gas segregation in open-conduit systems. However, the actual degassing mechanism of basaltic magmas at the origin of outstanding SO2 release is not satisfactorily explained by most of the geochemical models involving the magmatic redox conditions. It is commonly accepted that sulphur is transported exclusively as sulphide (SII-) or/and sulphate (SVI) by mantle-derived melts, before being released as SO2 and/or H2S in volcanic emissions [1].
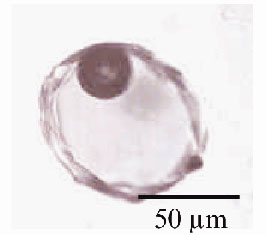 |
Fig. 41: Microphotograph of one olivine-hosted glass inclusion (Stromboli sample). The silicate melt is trapped at high temperature during the olivine crystal growth and preserved as a glass inclusion within the mineral, upon cooling. |
This work provides the first direct and quantitative determination of the oxidation state of sulphur in a selection of minute glass inclusions trapped in olivine crystals (Figure 41), using X-ray fluorescence microspectroscopy. The inclusions are representative of the variety of basaltic magmas that have supplied the activity of famous active volcanoes, e.g. Piton de la Fournaise (Reunion island), Stromboli and Mt Vesuvius (Italy). The micro-X-ray Absorption Near Edge Structure (XANES) experiments at the sulphur K-edge (2472 eV) were carried out using the X-ray microscopy beamline, ID21. The synchrotron X-ray source was demagnified to a micro-probe by using Fresnel zone plate lenses [2]. The spot size ranges from 0.5 x 0.5 to 2 x 2 µm2. At the sulphur K-edge, energy scans were provided by a fixed-exit Silicon (111) monochromator defining an energy resolution of 0.3 eV. The use of an X-ray fluorescence microprobe provides the required resolution in both energy and space together with an appropriate detection limit.
Figure 42 illustrates the micro-XANES spectra of the glass inclusions ((a) to (f)), and of one sulphate-bearing silicate glass, given for comparison. The spectra of inclusions indicate a clear predominance of sulphur dissolved as sulphide (SII-) in ocean-island basalts (Spectrum (a)). In contrast, they reveal the ubiquitous presence of sulphite (SIV) species in addition to sulphate (SVI) in inclusions representative of oxidised and water-rich basaltic magmas from subduction environments (Spectra (b) to (f)). The first peaks related to sulphite (SIV) and sulphate (SVI) are unequivocally identified at 2477.9 and 2482.1 eV, respectively, in each of these inclusions.
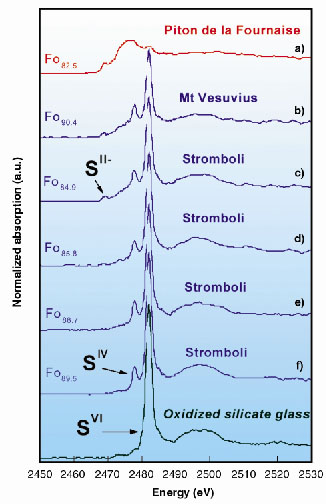 |
Fig. 42: Micro-X-ray Absorption Near-Edge Structure (XANES) spectra at the sulphur K-edge of the glass inclusions (a) to (f) and of one sulphate-bearing reference silicate glass. The sulphur concentration ranges from 950 to 2950 ppm. Fo refers to the composition of the olivine crystal which contains the inclusion [e.g., Fo89: [Mg/(Fe+Mg)] = 0.89]. |
Therefore, considering the equilibrium SO32melt = SO2gas + O2melt the decomposition of sulphite is a suitable candidate reaction to promote the release of sulphur as SO2 into the gas phase. The reduction of sulphate in sulphite and that of sulfite in SO2 may actually control the sulphur release into the gas phase upon ascent of H2O-rich oxidised basaltic magmas. We propose a new model involving sulphite (SIV) as the intermediate species yielding highly efficient partitioning of sulphur into the gas phase as the origin of the excess SO2 release in subduction zones. This mechanism may account for continuous sulphur release at open-conduit arc volcanoes, fed with water-rich basaltic magmas. Stromboli is one typical example of such volcanoes. A similar line of reasoning may apply to the pre-eruptive sulphur degassing of andesitic-type magmas and gas pressure build up in shallow reservoirs prior to major explosive eruptions.
References
[1] M.R.Carroll and J.D.Webster, in Volatiles in magmas, M.R. Carroll, J.R. Holloway (Eds.), Amer. Mineral. Soc., Washington D.C., 231-271 (1994).
[2] C. David, et al., App. Phys. Lett. 77, 3851 (2000).
Principal Publications and Authors
N. Métrich (a), M. Bonnin-Mosbah (b), J. Susini (c), B. Menez (a) and L. Galoisy (d), to be published.
(a) Laboratoire Pierre Süe, CNRS - CEA, CE-Saclay, Gif sur Yvette (France)
(b) INSTN/CFR, CEA, CE-Saclay, Gif sur Yvette (France)
(c) ESRF
(d) Laboratoire de Minéralogie-Cristallographie de Paris (France)



