- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Chemistry
- Chemical Interaction of Iron and Corundum at High Pressure and Temperature: Implication for the Earth's Deep Interior
Chemical Interaction of Iron and Corundum at High Pressure and Temperature: Implication for the Earth's Deep Interior
According to arguments based on cosmic abundance, Al2O3 is likely to be the next most abundant component in the Earth's lower mantle after MgO, FeO, and SiO2. Moreover, the D'' layer is possibly enriched in refractories such as Al2O3 and CaO. Therefore, the possible chemical reactions in the Fe-Al2O3 system can provide an important model for processes at the core-mantle boundary. It was demonstrated recently that even small amounts of Al can dramatically change the relative proportion of Fe3+ in (Mg,Fe)(Si,Al)O3 perovskite [1]. Consequently, the chemistry of the Fe-Al-O system is important for the whole Earth mantle. Knowledge of exchange reactions at high pressures and high temperatures between a metal from one side and refractory oxides and silicates from another side is important for understanding the early Earth differentiation. Therefore, there are a number of reasons to study interactions between aluminum oxide and iron at the megabar pressure range and high temperatures.
At ambient pressure corundum and iron do not react. At the same time, there are indications that such a reaction is possible at much higher pressures. However, so far, no systematic investigations of the interaction between iron and aluminum oxide at different pressures and temperatures have been performed and we decided to conduct in situ and ex-situ studies of the possible reactions between Fe and Al2O3 in electrically- and laser-heated diamond anvil cells (DAC) by means of X-ray powder diffraction on ID30, and Mössbauer spectroscopy analysis.
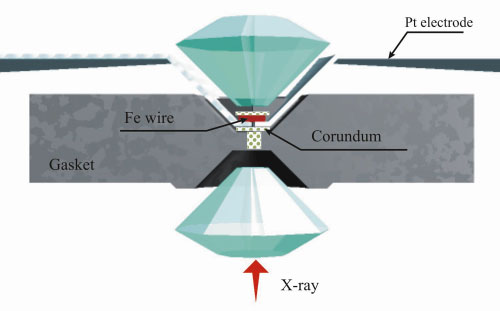 |
Fig. 34: Schematic diagram of the electrical heating assembly. A rhenium gasket of 250 µm thickness was indented to 25-30 µm between diamonds with 300 µm culets, and a hole of 100-110 µm in diameter was drilled in it. The gasket was covered by corundum-based cement and pure corundum was placed in the hole and around the wire. A platinum wire of 0.2 mm diameter flattened to a thickness of less than 10 µm was used as electrical leads. The iron wire was heated by a DC current with a stabilised power supply operating at 18 V/20 A range. |
In our experiments, a thin iron wire, 5 - 7 µm in diameter, was heated electrically (Figure 34) or by a Nd:YAG laser. At all pressures and temperatures up to 63(3) GPa and 1400(50) K, correspondingly, samples contained only the mixture of -Fe and corundum (Figure 35) after heating. However, at 65(3) GPa after heating at 2200(50) K, the diffraction pattern showed several rather weak additional reflections (Figure 35b). All these reflections could be interpreted as those belonging to the rhombohedral FeO phase. After decompression, the samples contained diffraction lines of
-Fe and cubic wüstite (Figure 35c). While the lattice parameters of corundum did not change after the experiment, the lattice parameter of iron increased. Moreover, the 110 reflection of
-Fe was slightly asymmetric from the side of higher d spacing (Figure 35c). Such changes in the diffraction pattern of iron correspond to the formation of Fe-Al alloy with approximately 2% Al by mass.
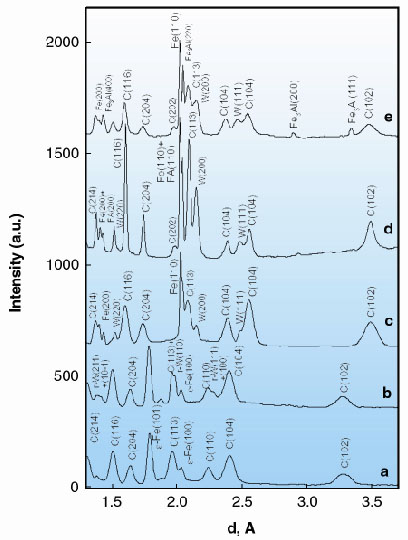 |
Fig. 35: Examples of X-ray diffraction patterns collected after heating iron and corundum at different pressures and temperatures: (a) at 63(3) GPa and 1400(50) K; (b) 65(3) GPa and 2200(50) K; (c) completely decompressed sample heated at 65(3) GPa and 2200(50) K; (d) and (e) different spots of the sample heated at 56(2) GPa and 2000(150) K. Diffraction patterns (a) to (c) of electrically-heated samples were collected at a laboratory X-ray source. (C, corundum; Fe, |
High-resolution synchrotron X-ray data can resolve the diffraction peaks from pure non-reacted iron (a = 2.8660(2) Å) and reacted iron alloyed with 3% Al (a = 2.8723(2) Å) (Figure 35d). Moreover, in one of the spots we observed additional reflections at 3.346 Å and 2.897 Å, which belong to cubic Fe3Al (a = 5.7946(4) Å) (Figure 35e).
Summarising the results of our experiments, we conclude that at pressures below 65 GPa and temperatures up to 2000 K corundum and iron do not react. At higher pressures and high temperatures we observed a chemical reaction which can be described in general as
(2 + 3x)Fe + xAl2O3 2FeAlx + 3xFeO,
where x is varied from 0.02-0.03 to 0.25.
The results show that iron is able to reduce aluminium out of oxides at the core-mantle boundary providing an additional source of light elements in the Earth's core and heterogeneity at the core-mantle boundary.
References
[1] C. A. McCammon, Nature, 387, 694-696 (1997).
Principal Publication and Authors
L. Dubrovinsky (a), N. Dubrovinskaia (a), H. Annersten (b), F. Westman (b), H. Harryson (b), O. Fabrichnaya (c) and S. Carlson (d), Nature, 412, 527-529 (2001).
(a) Bayerisches Geoinstitut, Universität Bayreuth (Germany)
(b) Department of Earth Sciences, Uppsala University (Sweden)
(c) Max Planck Institut für Metallforschnung, Stuttgart (Germany)
(d) ESRF



