- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Chemistry
- Metallisation of Halogens at High Pressures
Metallisation of Halogens at High Pressures
Diatomic molecules like H2, I2, Br2 or O2 can be considered as model systems to investigate the fundamental interactions in solids. In fact, through the application of pressure, the intermolecular interactions can be varied from those of the covalent diatomic molecule to the metallic ones of a compact three-dimensional solid. The molecular dissociation process has been observed in practically all diatomic molecules studied, with one remarkable exception, solid hydrogen. Thus, one can ask whether metallisation and dissociation are associated? For iodine, experiments showed that metallisation precedes dissociation, and for the other halogens (Br2, Cl2), for which the metallisation pressure has not been detected, models converge to the same idea. Nevertheless, strong discrepancies appear between the proposed metallisation mechanisms. Hence we decided to perform X-ray absorption (XAS) experiments at very high pressure in order to study metallisation in the case of bromine.
Experiments were performed at the ID24 beamline using diamond-anvil cells as pressure generators. The small sample size (50 µm in diameter) combined with the X-ray attenuation of the sample environment made it essential to exploit the high flux of the undulator with ultrafocusing dispersive optics. The pressure was measured in situ through the fluorescence of a ruby chip accompanying the sample, and a value of 110 GPa was attained, the highest ever reported in an XAS experiment. This has also been the first time that XAS at high pressure is used to provide evidence for a metallisation process.
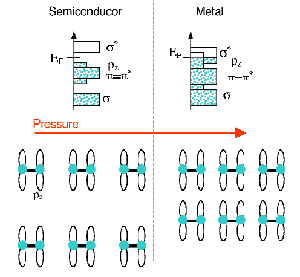 |
Fig. 14: (upper) Schematic band model for the metallisation of halogens; (lower) the evolution with pressure of two layers where the diatomic molecules form zig-zag chains.
|
The diatomic molecules of crystalline halogens lie in layers that interact through a very weak covalent interaction (pz-pz). During compression, the layers approach progressively and an enhancement of the pz-pz interaction widens the associated occupied band (Figure 14). In our model, metallisation takes place through progressive band closure when the pz band enters the empty ![]() * one (the band that derives from the broadening of the
* one (the band that derives from the broadening of the ![]() * level of the diatomic molecule). The
* level of the diatomic molecule). The ![]() * band also widens with pressure, but the introduction of the Fermi level at metallisation leads to a slower rate of pressure variation of the width of the empty states. Thus, metallisation can be detected through the evolution of the strong pre-peak of the bromine K-edge X-ray absorption spectrum (Figure 15) that corresponds to the empty
* band also widens with pressure, but the introduction of the Fermi level at metallisation leads to a slower rate of pressure variation of the width of the empty states. Thus, metallisation can be detected through the evolution of the strong pre-peak of the bromine K-edge X-ray absorption spectrum (Figure 15) that corresponds to the empty ![]() * band. Figure 15 shows that at a pressure of 25±5 GPa the expected change of slope is observed. Using the pressure evolution of the structure of bromine determined by X-ray diffraction, our calculations showed that this change can be consistently explained by the metallisation process. The value obtained for the metallisation pressure is well below some predictions, but in good agreement with the calculations of H. Miyagi et al. [1], confirming the metallisation mechanism here described. The phase transition associated with dissociation was also observed giving a discontinuous enlargement of the XAS pre-peak at a pressure of 65 ± 5 GPa.
* band. Figure 15 shows that at a pressure of 25±5 GPa the expected change of slope is observed. Using the pressure evolution of the structure of bromine determined by X-ray diffraction, our calculations showed that this change can be consistently explained by the metallisation process. The value obtained for the metallisation pressure is well below some predictions, but in good agreement with the calculations of H. Miyagi et al. [1], confirming the metallisation mechanism here described. The phase transition associated with dissociation was also observed giving a discontinuous enlargement of the XAS pre-peak at a pressure of 65 ± 5 GPa.
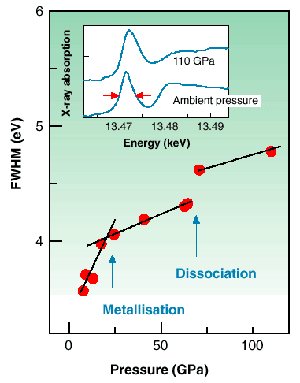 |
Fig. 15: Measured pressure dependence of the width of the Br K-edge pre-peak (empty
|
In conclusion, the mechanism of metallisation of halogens prior to dissociation has been clarified and an experimental value of 25 ± 5 GPa has been found for bromine. The possibility of an analogous sequence for hydrogen has recently been emphasised [2].
References
[1] H. Miyagi, K. Yamaguchi, H. Matsuo and K. Mukose, J. Phys. Condens. Matter, 10, 11203 (1998).
[2] K.A. Johnson, N.W. Ashcroft, Nature, 403, 632 (2000).
Principal Publication and Authors
A. San Miguel (a), H. Libotte (b), J.P. Gaspard (b), M. Gauthier (c), J.P. Itié (c), A. Polian (c), Eur. Phys. J., B17, 227-233 (2000).
(a) University of Lyon and CNRS (France)
(b) University of Liège (Belgium)
(c) University Paris VI and CNRS (France)



