- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- X-ray Imaging and Optics
- 2D mapping of texture in human dental enamel
2D mapping of texture in human dental enamel
Dental enamel is the most highly mineralised and strongest biological hard tissue. It comprises 95% hydroxyapatite (HA) mineral, 4% water, and 1% organic matter (non-collagenous protein). The hydroxyapatite crystal structure of dental enamel has been determined previously by several workers. It has space group P63/m with lattice parameters a = 9.513 Å and c = 6.943 Å [1,2]. However the measurements were made on powdered enamel collected from many teeth, therefore any texture information regarding the growth of the HA crystallites was lost. Information on texture is extremely valuable both for understanding the formation of dental enamel, and also to be able to increase the longevity of restorations by improving their clinical placement. In an earlier study Hirota examined the tilting of the enamel-prism orientation at twelve points in a human canine using laboratory 2D X-ray diffraction [3]. In our study, we have mapped, for the first time, the texture distribution as a function of position within entire slices of intact enamel using position-sensitive synchrotron X-ray diffraction. Here we present the preliminary results.
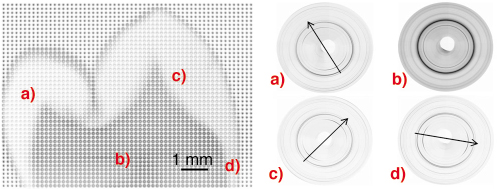
High-resolution synchrotron X-ray diffraction was used to collect 2D diffraction images at 150 µm spatial resolution over the entire tooth crown. Each diffraction image had an exposure time of 5s meaning that by moving the sample relative to the beam in an x and y direction a 150 µm-resolution map of the tooth on a grid of 10 mm x 7 mm could be collected in approximately eight hours. A 2D Mar CCD detector was used so that the change in texture around full diffraction rings could be observed. Basic texture distribution maps were generated by performing Rietveld refinement analysis of 1095 diffraction patterns, using an inhouse batch processing program and the GSAS software [4], and extracting the intensity (texture) coefficients. A composite map of CCD images of an adult second premolar is shown in Figure 131 where diffraction patterns were collected every 150 µm. Each point on the image is one 2D diffraction pattern, and these images have been arranged to form the whole image for illustration. The shape of the tooth can clearly be seen from this composite image. The lighter patterns around the edge are formed by the strongly textured enamel, and the darker patterns in the middle are the dentine. Below this image are four individual diffraction patterns from different parts of the tooth. Scans a), c) and d) illustrate the change in texture direction at difference positions within the enamel. Scan b) shows that dentine is nanocrystalline (broader peaks) and less textured.
 |
|
Fig. 131: 2D images of whole tooth section with different regions highlighted. a), c), d) enamel ; b) dentine. |
The strongest degree of preferred orientation was found in the 002 reflection, and areas of high crystallite alignment on the tooth cusps are found on the expected biting surfaces of the teeth. The texture direction of enamel is perpendicular to and follows the shape of the enamel-dentine junction. Additionally, it can be seen that below the groove between the two cusps (the fissure), there is a circular region of enamel which is less textured than the surrounding structure. We believe this to be caused by a fissure lesion in the enamel which has demineralised the enamel and possibly affected it’s crystalline structure.
By using spatially-resolved synchrotron X-ray diffraction, in a few hours of data collection, we have quantified the change in texture in dental enamel as a function of position within the tooth. Our study has been possible due to the high flux and energy (15 keV) of the synchrotron X-rays allowing counting times of a few seconds per image, and the use of a relatively thick sample to give us results representative of the bulk material. This has given detailed quantitative information on the degree of crystallite alignment in different regions of tooth enamel not previously reported. Our results bring novel insight on the texture distribution and crystallinity within teeth and have the potential to optimise the clinical placement of dental composite materials in restorations.
References
[1] R.A. Young, P.E. Mackie, Materials Research Bulletin 15, 17 (1980).
[2] R.M. Wilson, J.C. Elliott, S.E.P. Dowker, American Mineralogist 84, 1406 (1999).
[3] F. Hirota, Archives of Oral Biology 27, 931 (1982).
[4] A.C. Larson, R.B. Von Dreele, General Structure Analysis System (GSAS) Los Alamos National Laboratory Report, LAUR 86-748 (2004).
Principal Publication and Authors
M. Al-Jawad (a), R. Cywinski (a), S.H. Kilcoyne (b), D.J. Wood (c), R.C. Shore (c), L. Bouchenoire (d), A. Steuwer (e), Fifth International Conference on Synchrotron Radiation in Materials Science, Chicago July 30- Aug 2 (2006), SRMS5- 203. Conference Proceedings (full publication submitted to Biomaterials).
(a) School of Physics and Astronomy, Univ. of Leeds (UK)
(b) Institute for Materials Research, Univ. of Salford (UK)
(c) Leeds Dental Institute (UK)
(d) XMaS beamline, ESRF (France)
(e) FaME38, ILL-ESRF (France)



