- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- Soft Condensed Matter
- Structural Processes during Starch Granule Hydration by Synchrotron Radiation Microdiffraction
Structural Processes during Starch Granule Hydration by Synchrotron Radiation Microdiffraction
Starch granules are the most important energy reserve in higher plants. They are composed principally of amylopectin (major fraction) and amylose (minor fraction). Their structure is assumed to consist of concentric shells of alternating hard, semicrystalline, and soft amorphous layers. The starch granule organisation is, however, very complicated and many questions on the ultrastructure remain unsolved [1].
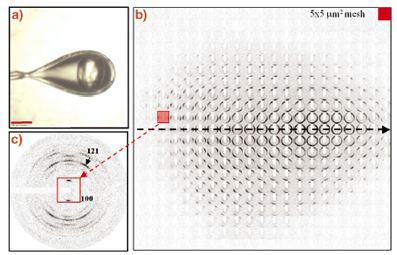
The microstructure of hydrated single potato starch granules had already been studied by X-ray microdiffraction in 1997 at beamline ID13 [2,3]. Radiation damage had, however, limited data collection to a few second per pattern [2]. Recent advances in microdiffraction techniques developed for biopolymers and protein microcrystals make revisiting this important biopolymer worthwhile. Figure 57a shows a single potato starch granule at 100 K in a cryoloop. A mesh-scan using a MAR CCD detector and a 5 mm beam is shown in Figure 57b with structural information in individual frames (Figure 57c) extending up to Q = 25 nm-1. These data suggest an increase in crystallinity upon hydration of the granule and confirm the model of shells with a radial orientation of amylopectin fibrils. Preliminary evidence for a crystallisation of short-range ordered amylopectin towards the granule centre was also obtained.
 |
|
Fig. 57: a) A single potato starch granule in a cryoloop at 100 K using a glycol/water cryoprotectant; b) 5 x 5 µm6 mesh-scan of single granule with a 5 µm beam. Note that the individual frames are rotated by 90°; c) single frame recorded in the outer parts of the granule showing a well- defined fibre diffraction pattern. |
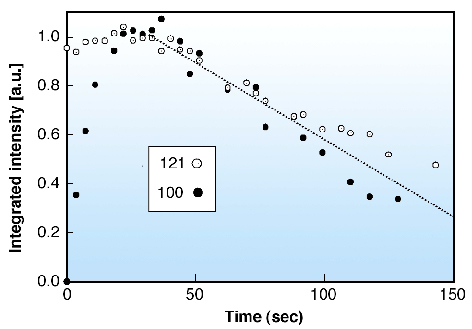
In order to advance the understanding of the structural steps involved in hydration and gelatinisation we studied the local hydration of single starch granules at room temperature by a drop-on-demand system using data collection times of 0.5 sec/frame. The time evolution of the 100 and 121 reflections is shown in Figure 58. Radiation damage is seen to be setting in for both reflections after the collection of 6-7 frames.
 |
|
Fig. 58: Kinetics of evolution of integrated intensities of the 100 and 121 reflections (see Figure 57c). Both reflections were scaled to 1.0 at maximum intensity. |
The 100 reflection is known to be particularly sensitive to hydration process [1] and suggests a half life of the hydration process of about 7 seconds. The unexpectedly rapid hydration reflects the porous nature of the starch granules [1]. The size of the crystalline domains was found to increase from 5.7 nm (unhydrated) to 9.4 nm (>10 sec hydration), which agrees well with the size of the proposed amylopectin side-clusters [1]. Larger domains of about 15 nm are only formed at longer hydration times. We plan to extend these experiments to combined SAXS/WAXS experiments and higher temperatures in order to study the onset of lamellar ordering and of gelatinisation.
References
[1] A. Buléon, P. Colonna, V. Planchot, S. Ball, Int. J. Biol. Macrom. 23, 85-112 (1998).
[2] A. Buléon, B. Pontoire, C. Riekel, H. Chanzy, W. Helbert, R. Vuong, Macromolecules 30(13), 3952-3954 (1997).
[3] T.A. Waigh, I. Hopkinson, A.M. Donald, M. Butler, F. Heidelbach, C. Riekel, Macromolecules 30(13), 3813-3812 (1997).
Principal Publications and Authors
H. Lemke (a), M. Burghammer (b), D. Flot (c), M. Rössle (d) and C. Riekel (b), Biomacromolecules 5(4), 1316-1324 (2004).
(a) Institut für Experimentelle und Angewandte Physik, University of Kiel (Germany)
(b) ESRF
(c) EMBL Outstation Grenoble (France)
(d) EMBL Outstation Hamburg (Germany)



