- Home
- News
- Spotlight on Science
- Synthesis of FeH5...
Synthesis of FeH5 under pressure: dense atomic metal hydrogen stabilised with Fe
27-07-2017
FeH5 was formed above 130 GPa in a laser-heated diamond anvil cell. It exhibits a remarkable layered structure trapping slabs of atomic hydrogen. This discovery of FeH5 suggests a low-pressure path to make materials that approach bulk dense atomic metal hydrogen.
Pure atomic metal hydrogen is expected to have remarkable properties such as room temperature superconductivity and other unprecedented quantum many-body properties, for example a superfluid-superconductor state. Despite continuous research over the past 40 years and many claims, atomic metal hydrogen has yet to be observed unambiguously.
Atomic metal hydrogen should be stable above 450 GPa. However, it is still a formidable experimental challenge to reach such a pressure and then to characterise the properties of a sample just a few µm in size. A decade ago, N. Ashcroft suggested that the high temperature superconductivity of atomic metal hydrogen should be observed in hydrogen dominant metallic alloys [1]. Due to the chemical pre-compression effect, the attainment of these metallic alloys should be within the usual capabilities of the diamond anvil cell.
This paper has stimulated an intense theoretical structural search. A novel view on the chemical combination of hydrogen with metals under pressure has been unveiled: the solubility of hydrogen in metals drastically increases under high pressure; hydrides of metals go from interstitial complexes to compounds formed with non-traditional H stoichiometries, dubbed super-hydrides. Many of the superconductivity temperatures calculated for these super-hydrides are indeed high, some even approaching ambient temperature. So far, only a few experimental studies have been devoted to superhydrides. Yet, the richness of this line of research has been recently confirmed by the discovery of superconductivity in hydrogen sulfide at a Tc of 203 K at 150 GPa, explained by the formation of H3S [2].
However, for high hydrogen concentrations, H atoms usually form complex hydrogenic sublattices composed of H-, H2, H3- and cages. It remains unknown whether a super-hydride could exist that is a real analogue of metal hydrogen, i.e. that exhibits a dense sublattice of hydrogen atoms. The discovery of FeH5 is the first observation of such an analogue of atomic metal hydrogen.
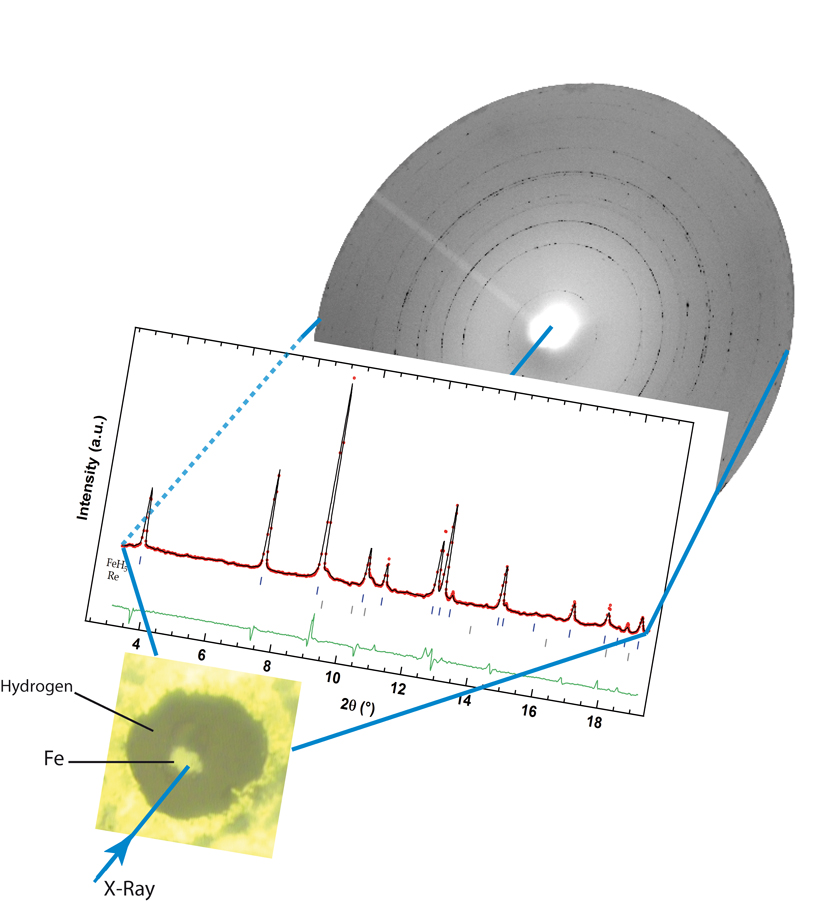
FeH5 was synthesised by a direct reaction of Fe and H2 above 130 GPa (a pressure of ~1.3 million atmospheres) in a diamond anvil cell. This compound consists of intercalated layers of quasi-cubic FeH3 units and of slabs of atomic hydrogen (see Figure 1). The ab initio calculations show that no bonding exists between these H atoms. The atomic H slabs interact with the Fe atoms to stabilise dense atomic H at much lower pressure than in pure hydrogen. FeH5 is a metal within its layers.
 |
|
Figure 1: Picture of the sample with the resulting X-ray diffractogram showing the Rietveld refinement of the tetragonal FeH5 structure. |
The experiments were performed at the ID27 X-ray diffraction beamline using an online laser-heating set up. Superhydrides were synthesised by compressing a little piece of metal in excess hydrogen and by laser heating it to ease the diffusion of hydrogen in the metal. In doing so, the hydride with the highest stoichiometry and stable at a given pressure could be observed. The difficulty of such an experiment is to avoid the chemical reaction of hydrogen with the sample chamber wall. A Fe polycrystalline sample, 2 microns thick and 10 microns in diameter, surrounded by a large amount of hydrogen, was loaded into a diamond anvil cell. The sample was insulated from the diamonds by 2-4 micron c-BN grains, to prevent heat loss through the diamonds and any parasitic chemical reactions with the diamonds. With hydrogen becoming highly reactive at high temperatures, the temperature has to be finely monitored and kept below ~1500 K to prevent any uncontrolled exothermic reaction from happening, which usually leads to diamond failure.
Between 3.5 and 125 GPa, the known interstitial stoichiometric compounds FeH, FeH2 and FeH3 were observed [3]. Above 130 GPa, laser heating of iron in excess of hydrogen allows for the synthesis of FeH5 and its equation of state was measured by decreasing the pressure down to 66 GPa, after which it decomposes back to FeH3. Rietveld refinement of the new X-ray pattern yields a tetragonal unit cell with symmetry I4/mmm (Figure 2). From the X-ray diffraction patterns, only the position of the Fe atoms can be inferred as the low scattering power of the hydrogen atoms prevents the determination of their position. However, using volume comparisons with ideal Fe-H solutions, it is possible to suggest a stoichiometry for the new compound: FeH5.
 |
|
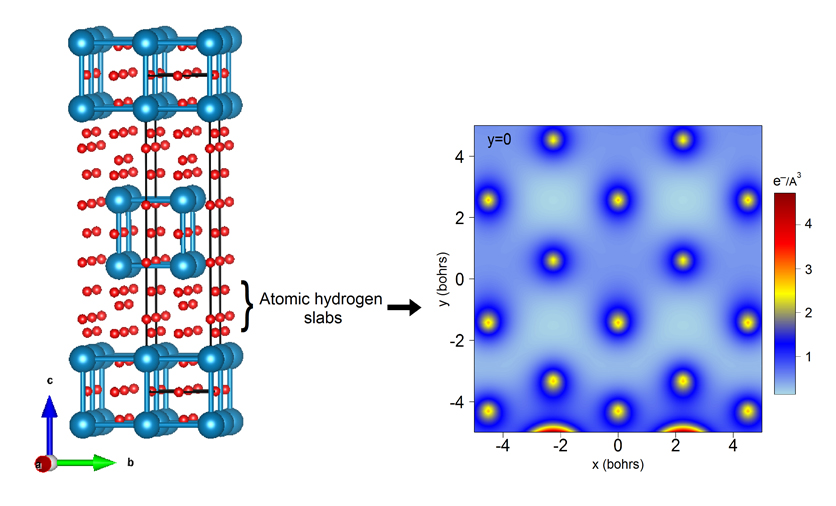
Figure2: (Left) Structure of FeH5 showing the presence of atomic hydrogen slabs. (Right) Electron density map along the c axis: no bonds between the hydrogen atoms can be found. |
Using this hypothesis, ab initio calculations were then performed to test all possible tetragonal structures with the determined Fe atoms positions and supporting the FeH5 stoichiometry. Among the 53 optimised structures, the one presented in Figure 2 has the lowest enthalpy and exhibits cell parameters in very good agreement with the ones measured experimentally.
These results show that the Fe-H system is an archetype of how hydrogen can combine with transition metals under pressure. At moderate pressures, structures consisting of interstitial hydrogen are formed. At higher pressure, slabs of atomic hydrogen can then be stabilised, thus suggesting a path towards bulk dense atomic hydrogen at low pressure. A little bit of Fe could do a lot to help unveil the intriguing properties of atomic metal hydrogen.
Principal publication and authors
Synthesis of FeH5: a layered structure with atomic hydrogen slabs, C. M. Pépin (a,b), G. Geneste (b), A. Dewaele (b), M. Mezouar (c), P. Loubeyre (b) Science 357, 382-385 (2017); doi: 10.1126/science.aan0961.
(a) CEA, Arpajon (France)
(b) EPSL, Institute of Condensed Matter Physics, EPFL, Lausanne (Switzerland)
(c) ESRF, Grenoble (France)
References
[1] N. W. Ashcroft, Phys. Rev. Lett. 92, 187002 (2004).
[2] A. P. Drozdov et al., Nature 525, 73 (2015).
[3] C. M. Pépin et al., Phys. Rev. Lett. 113, 265504 (2014).
Top image: Left: structure of FeH5 showing the presence of atomic hydrogen slabs. Right: electron density map where no bounds between the hydrogen atoms can be found.



