- Home
- News
- Spotlight on Science
- New type of charge-ordering...
New type of charge-ordering transition in the novel iron oxide Fe4O5
28-04-2016
Synthesis of new classes of compounds can lead to discoveries of novel physical phenomena as well as innovative applications. Fe4O5 is the first member of a very recently discovered family of iron oxides that can be synthesised only under high-pressure conditions. A multi-technique study of Fe4O5 reveals that it undergoes a unique charge-ordering transition below 150 K that involves competing dimeric and trimeric ordering within the chains of Fe ions. This electronic transition drives an intricate incommensurate distortion in its crystal structure.
At ambient pressure, only three iron oxide polymophs were known, FeO, Fe3O4, and Fe2O3. Recently, several new iron oxide polymorphs with hitherto unknown stoichiometries have been discovered under high pressure conditions [1,2]. These observations suggest the existence of a whole new class of iron oxide polymorphs yet to be discovered. Among them, Fe4O5 looks particularly exciting as it can be readily quenched at ambient pressure [1] and persists at ambient conditions as a metastable polymorph, thereby permitting detailed investigations of its physical properties and promising industrial applications.
We have performed a series of low-temperature studies using multiple probes on a recently-discovered high-pressure polymorph of iron oxide, Fe4O5 [1]. Fe4O5 has exhibited a new type of charge-ordering transition involving the formation of competing dimeric and trimeric ordering within the chains of Fe ions as revealed by X-ray studies at beamline BM01 (SNBL). These observations were also supported in neutron diffraction experiments and in measurements of magnetic and transport properties. To date, such exotic and puzzling transitions have never been documented for any other materials. Thus, the phase transition discovered in Fe4O5 brings new perspectives on charge-ordered states in mixed-valent iron compounds and, in general, presents a new prototype of charge-ordering-related transitions.
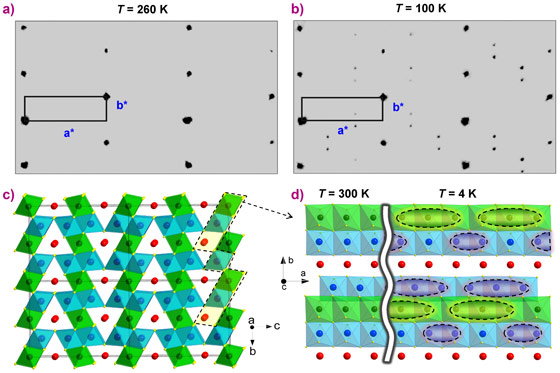
Under ambient conditions, Fe4O5 crystallises in a CaFe3O5-type crystal structure featuring linear chains of octahedrally-coordinated iron ions occupying two slightly different crystallographic positions, and linear chains of FeO6 trigonal prisms along the a-axis (Figure 1a,c). The compound contains equal amounts of Fe2+ and Fe3+ ions, and like another mixed-valent iron oxide, magnetite (Fe3O4), it is expected to be a good electrical conductor owing to a charge transfer between Fe2+ (charges) and Fe3+ (vacancies). Analysis of the bond valence sums in Fe4O5 suggested that octahedrally-coordinated iron ions have average charges of +2.76 and +2.66, whereas the cations in the prisms have an average charge of +1.92. In other words, the prisms are occupied by ferrous iron, while, the iron ions in the octahedral positions are in the mixed valence states.
Magnetite is known to undergo a charge-ordering phase transition below ~125 K at which point its electrical resistivity abruptly jumps by about two orders of magnitude [3]. This transition in magnetite had been discovered by Verwey in 1939 [3], but only recently, after more than 70 years, the elusive charge-ordering pattern in the low-temperature phase of Fe3O4 has finally been uncovered by means of single-crystal X-ray diffraction [4], and the charge ordering in magnetite was found to involve ‘three-site-distortions’, called ‘trimerons’ [4]. One could expect that Fe4O5 could also undergo some charge ordering at relatively low temperatures.
The high-quality single crystal X-ray diffraction data collected from Fe4O5 at beamline BM01 unambiguously demonstrated the appearance of superlattice reflections upon cooling below 150 K (Figure 1a,b). Indexing these superlattice reflections revealed that this structure is incommensurately modulated. The main features of this transition in Fe4O5 are the Fe dimers and trimers within the chains of the octahedrally-coordinated cations along the a-axis (Figure 1d). Furthermore, the chains of ferrous iron are only weakly modulated along the c-axis. A constant Fe2+-Fe2+ distance (~2.861 Å at 4 K) in the low-temperature structure served as a reference point, highlighting the dramatic shortening of some interoctahedra distances (marked by elongated ellipsoids in Figure 1d). Each chain of the octahedral cations contains either dimers or trimers, which is sketched in Figure 1d. The analysis of bond-valence-sums suggested that the trimers are composed of one Fe2+ and two Fe3+ ions, similar to the trimerons in Fe3O4 [3]. The Fe ions in the dimers have an oxidation state close to +2.5, i.e., the dimers are Fe2+–Fe3+ pairs with one shared electron.
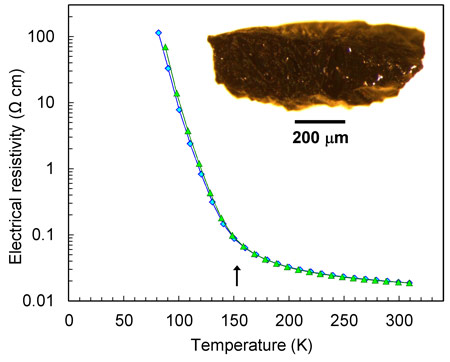
This unusual charge-ordering transition in Fe4O5 is concurrent with a significant increase in electrical resistivity (Figure 2), and therefore it may be classified as “metal-insulator” type. In addition, the magnetic susceptibility measurements and neutron diffraction establish the formation of a collinear antiferromagnetic order above room temperature and a spin canting at 85 K that gives rise to spontaneous magnetisation.
Principal publication and authors
Charge ordering transition in iron oxide Fe4O5 involving competing dimer and trimer formation, S.V. Ovsyannikov (a), M. Bykov (a,b), E. Bykova (a,b), D.P. Kozlenko (c), A.A. Tsirlin (d,e), A.E. Karkin (f), V.V. Shchennikov (f,g), S.E. Kichanov (c), H. Gou (a,h), A.M. Abakumov (i,j), R. Egoavil (i), J. Verbeeck (i), C. McCammon (a), V. Dyadkin (k), D. Chernyshov (k), S. van Smaalen (b), and L.S. Dubrovinsky (a), Nature Chemistry 8, 501–508(2016); doi: 10.1038/nchem.2478.
(a) Bayerisches Geoinstitut, Universität Bayreuth (Germany)
(b) Laboratory of Crystallography, Universität Bayreuth (Germany)
(c) Frank Laboratory of Neutron Physics, JINR, Dubna (Russia)
(d) National Institute of Chemical Physics and Biophysics, Tallinn (Estonia)
(e) Institute of Physics, University of Augsburg (Germany)
(f) Institute of Metal Physics, Russian Academy of Sciences, Yekaterinburg (Russia)
(g) Institute for Solid State Chemistry, Russian Academy of Sciences, Yekaterinburg (Russia)
(h) Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao (China)
(i) Electron Microscopy for Materials Research (EMAT), University of Antwerp (Belgium)
(j) Department of Chemistry, Moscow State University (Russia)
(k) Swiss–Norwegian Beamlines at the ESRF, Grenoble (France)
References
[1] B. Lavina, et al. Proc Nat. Acad. Sci. US 108, 17281 (2011).
[2] E. Bykova, et al., Nature Commun. 7, 10661 (2016).
[3] E.J.W. Verwey, Nature 144, 327 (1939).
[4] M.S. Senn, et al. Nature 481, 173 (2012).
Top image: Crystal structure of Fe4O5 at low temperature showing dimeric and trimeric ordering.