- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- X-ray imaging
- Recovering the firing protocol of ancient ceramics through iron phase distribution analysis using full-field XANES imaging
Recovering the firing protocol of ancient ceramics through iron phase distribution analysis using full-field XANES imaging
Fine ceramics with black high gloss slip on red bodies, manufactured in the Mediterranean basin from 6th c. BCE to 1st c. CE, were a benchmark of technological innovation in high temperature redox chemistry [1]. This technology, first developed in Corinth and Athens (6th c. BCE), slowly moved west to central and northern Italy (4th c. BCE) and finally to southern Roman Gaul where it vanished in the early decades of the 1st century CE. Although several tens of thousands of ceramic fragments have survived, a detailed technological protocol on how they were produced is almost entirely lost. Here, using two black gloss sherds from the Roman period, we show that by probing their mineralogical variation and distribution we can reconstruct some of this lost technology. Our results also indicate that although the basic Fe redox chemistry remained unchanged for over 600 years, significant changes in firing protocol took place even over a span of less than 100 years [2].
Because the minerals that reveal the lost processing conditions are often very heterogeneously distributed and confined to near surface and interfacial regions between the ceramic body and the slip, a probe with high spatial resolution, large field of view and high sensitivity to mineralogy is required to map them.
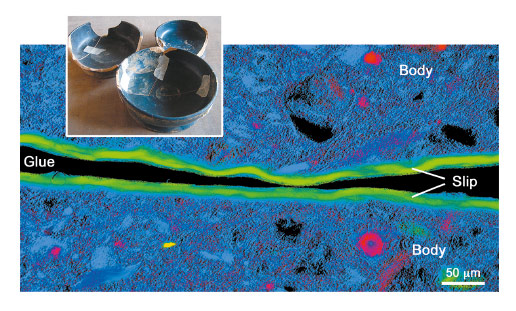
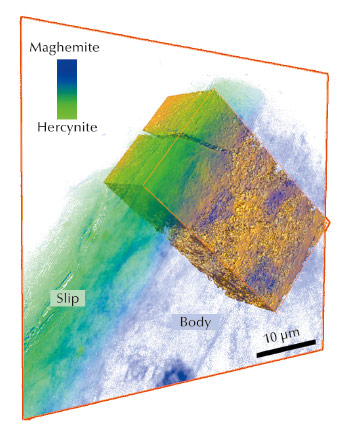
A new full-field spectromicroscopy technique at beamline ID21 [3] that combines XANES and full-field imaging allowed us to map the Fe-phases in a Campanian ceramic (1st c. BCE, Italy), see Figure 21, and a similar microscope at BL 6-2 SSRL [4] allowed us to correlate Fe mineralogy with 3D porosity for another fragment from the same sherd. The phase maps show that dense zones (slip and some body regions) are hercynite (Fe2+, green in Figure 21), while more oxygen permeable regions were predominantly maghemite (Fe3+, blue in Figure 21). Similar phase maps, not shown, were obtained for a Pre-sigillata ceramic (1st c. CE, Roman France). The mineralogical maps from the two sherds show subtle but significant differences.
 |
|
Fig. 21: Campanian ceramics (1st c. BCE) and Fe phase map of the cross-section of a sample. Hercynite is shown in green, maghemite in blue, hematite in red and almandine in yellow. |
The presence and distribution of maghemite, an intermediate Fe+3 mineral formed during re-oxidation of hercynite, is the key to some of the firing protocols. Occurrences of maghemite at the outside surface and the interface between slip and body, and especially the tendril like incursion from oxidised to reduced areas visible in Figure 22, in both ceramics is compelling and very pictorial evidence that these vessels were once reduced and then partially re-oxidised. Furthermore, a relatively uniform hercynite slip and nearly absent re-oxidation layer on the surface indicates a successful firing protocol that allowed the body to re-oxidise while preventing most of the slip from doing so. This remarkable achievement, that would be difficult even in modern replications, suggests possession of great skill in controlling the redox chemistry through sophisticated manipulation of clay chemistry and morphology and kiln firing conditions.
 |
|
Fig. 22: Registration of the 3D tomographic data with the 2D mosaic phase map of the cross-section Campanian sample. |
Differences in maghemite/hematite distribution in the two sherds, on the other hand, point to differences in firing protocol by highlighting two critical differences. The first critical difference is the very patchy and, when present, very thin oxidation layer on the surface of the Campanian slip in contrast to a thicker and more uniform layer on the Pre-sigillata slip. The second is the absence of hematite, except for a few small islands centred on embedded crystals, in the body of Campanian sherd as opposed to mostly hematite body of the Pre-sigillata sherd. These two distinctions, when combined with the presence of patches of un-oxidised Fe+2 minerals in the body (Almandine particle, and small patches of hercynite) suggests that the final re-oxidation step (in the three phase firing protocol, first outlined by Noble [1]) for the Campanian ceramic must have occurred at significantly lower temperature and/or shorter duration than that for the Pre-sigillata ceramic.
Full-field XANES microscopy should have a profound impact on the recovery of lost ancient ceramic technology. Its versatility should also lead to a deeper understanding of the functioning of other complex, hierarchically heterogeneous systems, such as heterogeneous catalysis and energy storage systems.
Principal publication and authors
F. Meirer (a), Y. Liu (b), E. Pouyet (c), B. Fayard (c), M. Cotte (c, d), C. Sanchez (e), J.C. Andrews (b), A. Mehta (b) and P. Sciau (f), J. Anal. At. Spectrom. 28, 1870-1883 (2013).
(a) Debye Institute for Nanomaterials Science, Utrecht (Netherlands)
(b) SSRL-SLAC, Menlo Park (USA)
(c) ESRF
(d) Laboratoire d’Archéologie Moléculaire et Structurale, CNRS, Ivry-sur-Seine (France)
(e) Archéologie des Sociétés Méditerranéennes, CNRS, Lattes (France)
(f) CEMES-CNRS, Toulouse (France)
References
[1] J.V. Noble, The techniques of painted Attic pottery, New York (1965).
[2] P. Sciau, Y. Leon, P. Goudeau, S.C. Fakra, S. Webb and A. Mehta, J. Anal. At. Spectrom. 26, 969 (2011).
[3] B. Fayard, E. Pouyet, G. Berruyer, D. Bugnazet, C. Cornu, M. Cotte, V. De Andrade, F. Di Chiaro, O. Hignette, J. Kieffer, T. Martin, E. Papillon, M. Salomé and V. A. Sole, Journal of Physics: Conference Series 425, 192001 (2013).
[4] F. Meirer, J. Cabana, Y.J. Liu, A. Mehta, J.C. Andrews and P. Pianetta, Journal of Synchrotron Radiation 18, 773 (2011).



