- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Dynamics and extreme conditions
- New structures of carbonates at high pressures and their relevance for the deep carbon cycle
New structures of carbonates at high pressures and their relevance for the deep carbon cycle
Calcite, CaCO3, and dolomite, CaMg(CO3)2 are two very common minerals on the Earth’s surface. They are probably formed in the deep sea sediments and coral reefs. The chemical equilibria regulating their precipitation is recognised as being affected by the CO2 content in the oceans and atmosphere, two parameters which are directly related to the Earth’s actual and past climate [1]. Nevertheless, not all the marine carbonate sediments are transformed into limestone and preserved at the Earth’s surface because a significant portion of the oceanic carbonate sediments are transferred into deep Earth through the subduction mechanism of the oceanic crust and its overlying sediment layers. Therefore, in order to understand the whole Earth carbon cycle, it is fundamental to take into account the transformation of carbonates within the Earth’s mantle as well. In such a way, carbon may be transported and stored at depth, and it may also return back to the Earth’s surface through volcanic processes. The first step in any of these investigations, however, is assessing the structures of these carbon-rich materials at extreme conditions.
Here we focussed in particular on the phase behaviour of dolomite, in order to study the phase evolution at the high pressures and temperatures expected in the Earth’s mantle. Very tiny single crystal samples (20x20x5 µm3) were pressurised in diamond anvil cells up to 80 GPa, and the structure evolution of dolomite was monitored by X-ray single crystal diffraction.
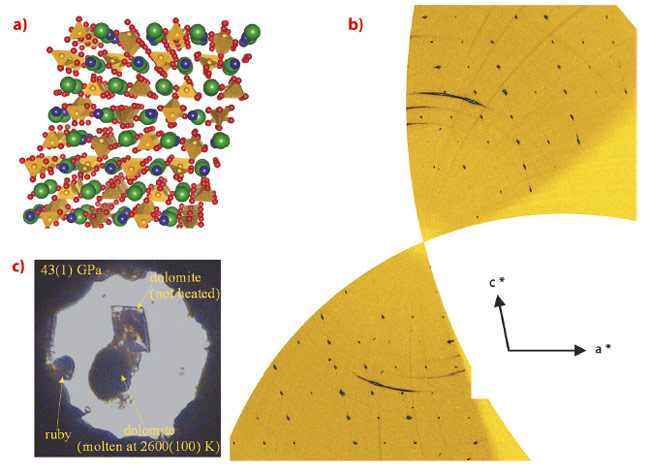
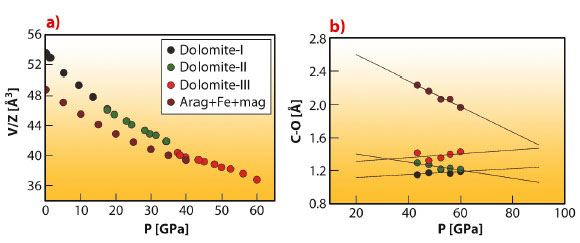
The experiments were performed at beamline ID09A, employing the recently optimised single-crystal diffraction setup, well-suited to the investigation of samples as small as a few µm3 in size and to pressures in excess of a megabar. Dolomite undergoes two structural transitions above 17 GPa (dolomite-II) and 35 GPa (dolomite-III), respectively. The first one is a displacive transition towards a lower symmetry structure, very similar to the one encountered in CaCO3 at much lower pressures [2]. The density of dolomite-II (triclinic, a = 4.7407(10) Å, b = 5.3885(10) Å, c = 6.7430(10) Å, α = 101.42(1)°, β = 89.27(1)°, γ = 95.72(1)°, V =168.01(5) Å3) is similar to dolomite-I. The second transition (Figure 35) exhibits a first-order character, towards a different triclinic phase (a = 6.2346(9) Å, b = 9.3025(11) Å, c = 10.9893(12) Å, α = 75.89(1)°, β = 81.05(1)°, γ = 89.48(1)°, V = 610.32(14) Å3). Despite the significant volume changes (5%) upon transition (Figure 36), a single-crystal domain with quality suitable for structural investigation was preserved.
 |
|
Fig. 36: a) Volume behaviour of dolomite, dolomite-II and dolomite-III, compared to the volume of a chemically-identical mixture of aragonite (the high pressure polymorph of CaCO3) and Fe-magnesite (Mg,Fe)CO3. b) Evolution of C-O distances as a function of pressure for selected C atoms. The presence of a fourth C-O chemical bond that progressively shrinks as a function of pressure is particularly evident, pointing towards a tetrahedrally coordinated carbon at extreme pressures. |
The structure was solved with the charge-flipping method and Fourier analysis and was found to consist of 80 atoms in the unit cell. The main structural features are the presence of non-coplanar CO3 groups, a feature also present in the recently solved CaCO3-III structure [3]. Moreover, the presence of a further chemically bonded oxygen close to the carbon positions (Figure 36) indicates that this structure represents an intermediate topology between the low pressure carbonates and the predicted ultrahigh pressure carbonates, with tetrahedral coordinated carbon. The thermodynamic stability of dolomite-III at combined ultrahigh pressures and temperatures was investigated using the recently developed laser heating system for single crystal diffraction. These results indicate that single-phase dolomite-III is stable at the high pressures and temperatures existing in the lower mantle, also in agreement with another recent experimental study [4]. Dolomite-III may therefore act as an important carbon repository in the lowermost mantle. The similarity between dolomite-III and calcite-III even suggests the possibility of an extended solid-solution between CaCO3 and CaMg(CO3)2, with this new structure acting therefore as the main vehicle for carbon transport in subduction environments, where experimental investigations reveal that a Mg-calcite, a composition intermediate between the calcite and dolomite compositional join, is the main carbon-bearing phase in multicomponent systems representative of the chemistry of the oceanic crust and sediment layer.
Principal publication and authors
M. Merlini (a), W.A. Crichton (b), M. Hanfland (b), M. Gemmi (c), H. Müller (b), I. Kupenko (d) and L. Dubrovinsky (d), Proc. Natl. Acad. Sci. U.S.A. 109, 13509-13514 (2012).
(a) Dipartimento di Scienze della Terra, Università degli Studi di Milano (Italy)
(b) ESRF
(c) Center for Nanotechnology Innovation@NEST, Istituto Italiano di Tecnologia, Pisa (Italy)
(d) Bayerisches Geoinstitut, Universität Bayreuth (Germany)
References
[1] A. Ridgwell and R.E. Zeebe, Earth Planet. Sci. Lett. 234, 299–315 (2005).
[2] L. Merrill and W.A. Bassett, Acta Cryst. B31, 343–349 (1975).
[3] M. Merlini, M. Hanfland and W.A. Crichton, Earth Planet. Sci. Lett. 333–334, 265–271 (2012).
[4] Z. Mao et al. Geophys Res. Lett. 38, L22303 (2011).