- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Dynamics and extreme conditions
- Nuclear resonance scattering enlightens binding of cardiovascular regulatory NO to heme proteins
Nuclear resonance scattering enlightens binding of cardiovascular regulatory NO to heme proteins
The diatomic molecule nitric oxide, NO, plays an essential role as a signal molecule for cardiovascular regulation, the regulation of cell function and as a neurotransmitter in vertebrates, as well as a toxic defence substance for eliminating invading organisms [1]. NO is toxic in high concentration, its production and transport are strongly regulated. Insects such as the Amazon river-based kissing bug Rhodnius prolixus have developed the strategy of causing vasodilation by injecting the suite of NO-transporter heme proteins, the nitrophorins (NP), into the tissues and capillaries of their victims [2]. NO dissociates upon dilution and pH rises from ~5−6 in the salivary glands of the insect to ~7.3−7.4 when the saliva is injected into the victim’s tissues.
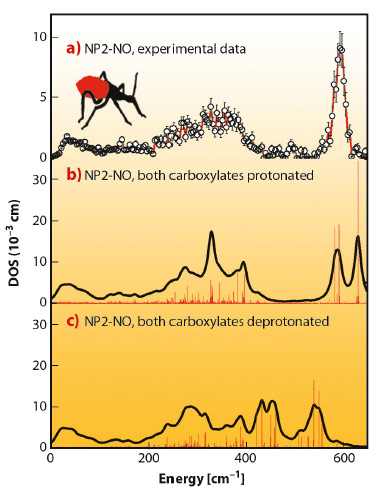
We have found that nuclear inelastic scattering (NIS) could not only pin down heme NO binding modes in nitrophorins, but also possibly functionally relevant low-energy modes at energies down to 10 cm−1, and all this in one experiment. The experimental iron vibrational density of states (DOS) shown in Figure 53a exhibits a broad dominant peak around 600 cm−1 and has also been calculated by quantum mechanical (QM) density functional theory (DFT) coupled with molecular mechanics (MM) methods. The simulations identify this dominant peak as caused by iron-NO binding modes with major stretching character. The simulation shown in Figure 53b was performed with the heme carboxylates of NP2–NO both being protonated. This was motivated by the low pH of the NP2–NO sample (pH 5.5), which was chosen to be similar to that of the saliva of R. prolixus. The calculations in Figure 53c show that deprotonation of the heme carboxylates significantly reduces the iron-NO stretching frequencies. This indicates an unexpected effect, namely a weakening of the iron-NO bond, which might occur even in vivo when, after being bitten, the nitrophorins are injected into the neutral pH blood of the victim.
 |
|
Fig. 53: Iron vibrational density of states (DOS) for NP2−NO: a) experimental data, b) simulation with both heme carboxylates protonated, and c) with both heme carboxylates deprotonated. Reprinted with permission from B. Moeser et al. J. Am. Chem. Soc. 134, 4216. Copyright (2012) American Chemical Society. |
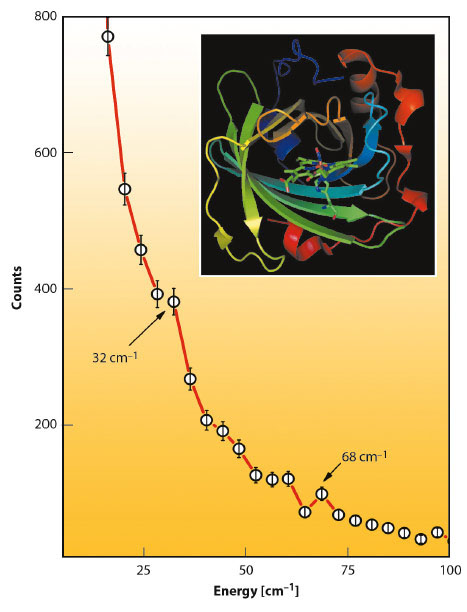
At energies below 100 cm−1 about 300 protein modes have been calculated. Among the most intense are 4 modes around 70 cm−1 all of which involve considerable heme doming. It is also striking that the experimental NIS spectrum of NP2-NO has a band at 68 cm−1 (Figure 54). The remaining three modes with the highest iron displacement below 100 cm−1 are at 17, 36, and 29 cm−1. These modes represent mainly a heme sliding motion [3], which is around 36 cm−1 coupled to a twisting of the β-barrel subunits. Indeed, there is also an experimentally observed band at around 32 cm−1. These low-energy heme modes are believed to be functionally relevant and may support NO capture and release, not only in nitrophorins but also in other heme proteins as well.
 |
|
Fig. 54: Low-energy region of the raw NIS data of NP2-NO with indicated peaks caused by translational heme modes (32 cm−1) and by heme doming modes (68 cm−1). |
The presented results may be important with regard to a potential application of these type of NO carrier proteins and also other heme-containing systems such as cardiovascular drugs. The binding of NO to heme iron can obviously be affected by the pH-value of the fluid environment, an effect which has not been considered before.
Principal publication and authors
B. Moeser (a), A. Janoschka (a), J.A. Wolny (a), H. Paulsen (b), I. Fillipov (c), R.E. Berry (c), H. Zhang (c), A.I. Chumakov (d), F.A. Walker (c) and V. Schünemann (a), J. Am. Chem. Soc. 134, 4216 (2012).
(a) Department of Physics, University of Kaiserslautern (Germany)
(b) Institute of Physics, University of Lübeck (Germany)
(c) Department of Chemistry and Biochemistry, University of Arizona, Tucson (USA) (d) ESRF
References
[1] S. Moncada, R.M.J. Palmer and E.A. Higgs, Pharmacol. Rev. 43, 109-142 (1991).
[2] J.M.C. Ribeiro, J.M.H. Hazzard, R.H. Nussenzveig, D.E. Champagne and F.A. Walker, Science 260, 539-541 (1993).
[3] K. Achterhold and F.G. Parak, J. Phys: Condens. Matter 15, S1683-S1692 (2003).



