- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Dynamics and extreme conditions
- Cold melting and solid structures of dense lithium
Cold melting and solid structures of dense lithium
The alkali metals, due to their simplicity, have attracted the attention of physicists and chemists alike for more than hundred years. They have been used by scientists to create the Bose-Einstein condensates and to develop the theory of the solid state. Undoubtedly, there are still more surprises to be discovered with the alkali metals.
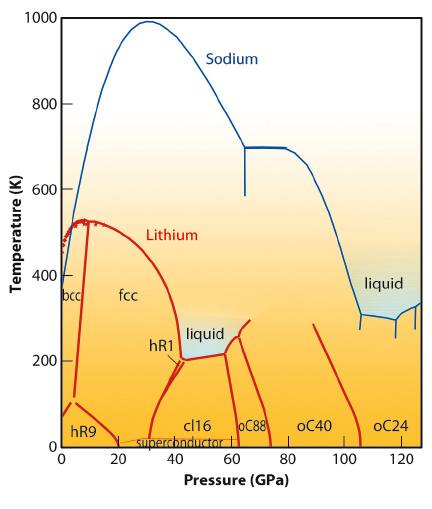
Our investigation focuses on how light alkali (Na and Li) melt when their density is greatly increased. We have discovered an unprecedented pressure-induced drop in the sodium melting temperature from 1,000 K at 30 GPa (30,000 Atm) down to room temperature at 120 GPa (1.20 MAtm) [1] (see also Figure 7) – a highly unusual and contra-intuitive result. This study was inspired by the prediction that at very high pressures, currently un-attainable in laboratory, hydrogen will be metallic and liquid even at T=0 K, a novel quantum state of matter never observed before.
 |
|
Fig. 7: Phase diagram of lithium (red) and sodium (blue). The lithium phase diagram indicates the various solid states and also the liquid state at 50 GPa (0.5 Mbar) and temperatures below 200 K. |
Since the sodium atom is too heavy to be a quantum system, we have turned our attention to its lighter counterpart – lithium. At low pressure and very low temperatures, lithium forms rhombohedral crystals that, at higher pressures and temperatures, transform to face-centred cubic and then body-centred cubic crystals – all three among the simplest known crystal structures. However, what happens at very high pressures? In the past few years, intriguing deviations from simple metallic behaviour were observed, for example a metal to semiconductor-transition and even superconductivity at 17 K. In our study, we found even more surprises: above 60 GPa, lithium adopts three novel, complex crystal structures with 40, 88 and 24 atoms per unit cell that were not previously observed in any element (see Figure 7). The most complex structures with 40 and 88 atoms had never even been predicted theoretically. Interestingly, the samples in these phases were becoming progressively darker with pressure, indicating profound changes in their electronic structure. This prompted us to conduct a joint theoretical and experimental study in which we have shown that lithium is a semiconductor with a band-gap of 1 eV [2].
The melting of lithium under pressure also revealed some unexpected results. While the melting point of a material usually rises with pressure (besides Na), and even the lightest gaseous elements, hydrogen and helium, melt at 1000 K and 50 GPa, lithium remains liquid at this pressure down to temperatures as low as 190 K. This is by far the lowest melting temperature observed for any material at this pressure.
One of the possible explanations of the overall appearance of the lithium phase diagram, and particularly of the anomalously low melting temperatures, is that quantum effects are starting to play the dominant role at high compressions. However, further investigations, particularly theoretical calculations, are needed to clarify this. We also speculate that a ground metallic liquid state, which has been predicted but never observed for hydrogen and which should exhibit highly unusual properties, might be constructed on the basis of the lithium-rich compounds e.g. the combination of lithium with light gases such as hydrogen or helium.
Principal publication and authors
C.L. Guillaume (a), E. Gregoryanz (a), O. Degtyareva (a), M.I. McMahon (a), M. Hanfland (b), S. Evans (b), M. Guthrie (c), S.V. Sinogeikin (d) and H-K. Mao (c,d), Nature Physics 7, 211-214 (2011).
(a) SUPA, School of Physics and Astronomy, and Centre for Science at Extreme Conditions, The University of Edinburgh (UK)
(b) ESRF
(c) Geophysical Laboratory, Carnegie Institution of Washington, Washington D.C. (USA)
(d) HPCAT, Carnegie Institution of Washington, Argonne (USA)
References
[1] Gregoryanz et al., Phys. Rev. Lett. 106, 185502 (2005).
[2] Marques et al., Phys. Rev. Lett. 106, 095502 (2011).



