- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Structural biology
- Assembly and channel-opening in a bacterial drug efflux machine
Assembly and channel-opening in a bacterial drug efflux machine
Resistance to drugs remains a major obstacle in the treatment of infections caused by pathogenic bacteria. One drug resistance mechanism originates from the activity of transmembrane transporters that displace a broad range of antibiotics and other toxic compounds from the bacterium. In the case of Gram-negative bacteria, the toxic compound may need to be displaced across both the inner- and outer-membranes and the interstitial periplasm in order to be properly expelled from the cell. A special class of three-component efflux pumps accomplishes this task. Typically, these pumps are composed of an inner membrane protein, an outer membrane protein, and a protein that traverses the periplasm to link the two membrane proteins.
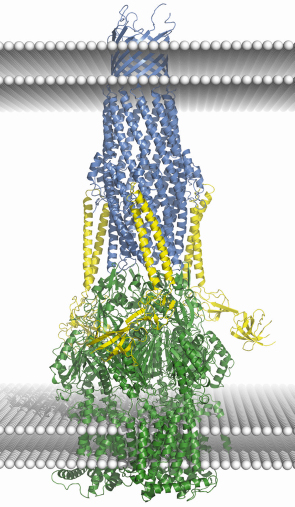
A well studied representative of the family of multi-drug efflux pumps is the Escherichia coli AcrB/AcrA/TolC assembly. Crystal structures are available for each of the three components of the tripartite assembly [1, 2 and references cited therein], but the structure of the ternary assembly is presently unknown. Accumulating structural and functional data provides a rough idea of the architecture of the pump, and Figure 77 summarises these in a speculative model for the entire assembly based on docking the crystal structures of the individual components. The trimeric AcrB is the inner membrane component, and it powers the efflux pump using the energy from proton electrochemical gradients. The trimeric TolC is the outer membrane protein, which is shaped like a cylinder with a hollow interior. One end of the cylinder is open to the extracellular medium while the other tapers towards its periplasmic end, where it is maintained in a closed state by salt bridges and van der Waals contacts between the adjacent subunits. It seems clear that transport requires TolC to switch to an open conformation to allow transportation of the substrate.
 |
|
Fig. 77: A cartoon schematic showing a putative model of the AcrB/AcrA/TolC drug-efflux assembly. Crystal structures of the isolated components were docked to model the assembly. The TolC trimer is shown in blue, AcrB trimer in green, three AcrA molecules are coloured yellow. White sphere layers represent the two surfaces of each membrane; the two upper and two lower are from the outer membrane and inner membrane respectively. While the model presented here depicts a 3:3:3 assembly, other models, with different subunit compositions cannot be ruled out by the available data. |
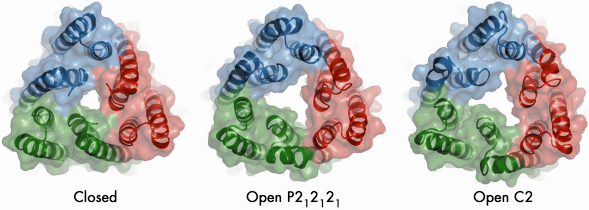
In our study, we mutated TolC in order to disrupt a network of hydrogen bonding interactions at the periplasmic end that caused it to become leaky to bulky molecules in vivo. However, the mutations did not affect TolC function, since it retained the capacity to form a functional efflux assembly. We solved the crystal structure of the mutant in two crystalline forms at 3.2 and 3.3 Å resolution using data collected at beamline ID23-2. The crystal structures reveal that three of the six pairs of coiled coils at the closed end of TolC move radially outward from the central molecular axis to partially open the channel (Figure 78). Conformational changes associated with partial channel opening expose grooves which, we speculated, can accommodate periplasmic component AcrA. We developed a model of the interactions between AcrA and TolC, which successfully predicted a mutation pair in the proteins that compensate each other’s disruptive effects. Using docking calculations, we generated a model in which the open form of TolC engages the periplasmic crown of AcrB, the inner membrane protein.
 |
|
Fig. 78: Crystal structures of the open and closed states of the TolC channel protein. The view is along the three-fold axis of the TolC trimer at the periplasmic end that engages AcrB. |
Despite the dramatic changes in the AcrB during its work cycle [1,2], the TolC-interacting interface of AcrB changes little, suggesting that this interface might only partially transmit the conformational changes that trigger the opening of the TolC channel, and is likely only implicated in unlocking the periplasmic end of the channel, while additional energy is needed to drive the channel to fully open state. This suggests that AcrA is more than just a passive bridge between the energised AcrB and TolC, but likely also serves as an active transducer of energy from one to the other. The induced fit of the periplasmic partner into the partially opened grooves of TolC may complete the process of channel opening.
Principal publication and authors
V.N. Bavro (a), Z. Pietras (a), N. Furnham (a), L. Pérez-Cano(b), J. Fernández-Recio (b), X. Yuan Pei (a), R. Misra (c) and B. Luisi (a), Mol. Cell. 30, 114 (2008).
(a) Department of Biochemistry, University of Cambridge (UK)
(b) Barcelona Supercomputing Center (Spain)
(c) Arizona State University, Tempe (USA)
References
[1] S. Murakami, R. Nakashima, E. Yamashita, T. Matsumoto and A. Yamaguchi, Nature 443, 173 (2006).
[2] M.A. Seeger, A. Schiefner, T. Eicher, F. Verrey, K. Diederichs and K.M. Pos, Science 313, 1295 (2006).



