- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2000
- Materials
- Study of MgO-FeO Solid Solution at Conditions of Earth's Lower Mantle
Study of MgO-FeO Solid Solution at Conditions of Earth's Lower Mantle
Knowledge of the dynamics of the Earth's mantle is important for understanding the global changes in the history of our planet. Ferropericlase (Mg,Fe)O and (Mg,Fe)SiO3-perovskite are considered the constituents of the bulk of the Earth's lower mantle. Therefore, ferropericlase and magnesiowüstite have been studied extensively. At ambient condition, the end members of the MgO-FeO solid solution - periclase (MgO) and wüstite (FeO) - have the same halite NaCl (B1) structure and they form a complete solid solution (Figure 99). However, at pressures above 17 GPa and ambient temperature, wüstite transforms into a phase with rhombohedral structure (Figure 99). With increasing pressure above 100 GPa at 300 K it transforms to the NiAs (B8) or the anti-NiAs (a-B8) structure [1], whereas periclase retains the NaCl structure at least to 227 GPa [2]. The NaCl structure is based on cubic close packing of the anions, whereas the B8 (or the a-B8) structure is formed by hexagonal close packing of the anions (or cations) (Figure 99). The topological difference between the B8 and the B1 structure at high pressure could lead to an immissibility gap in the region of the MgO-FeO solid solution. To investigate this hypothesis we conducted an in situ high-P,T study of ferropericlase with intermediate compositions, (Mg0.6Fe0.4)O, and quenched (Mg0.5Fe0.5)O and (Mg0.8Fe0.2)O, respectively.
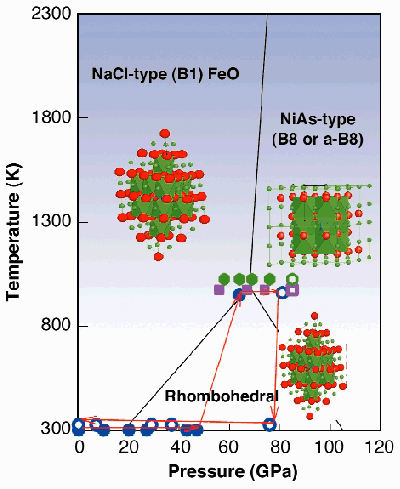 |
Fig. 99: Pressure and temperature conditions of the experiments. Solid symbols correspond to initial (cubic) magnesiowüstite phase, open symbols to mixture of two cubic phases in quenched samples. Circles are for (Mg 0.6Fe 0.4)O, squares are for (Mg 0.5Fe 0.5)O, and hexagons are for (Mg 0.8Fe 0.2)O. Lines represent phase boundaries between wüstite phases (1).
|
Experiments were performed on beamline ID30. We collected the powder diffraction data with a fine incident X-ray beam (approximately rectangular shape with dimensions 8*9 mm2 or less) of 0.3738 Å wavelength on the FastScan imaging plate.
An example of our experiments with ferropericlase (Mg0.6Fe0.4)O follows: first we increased the pressure to 47 ± 1 GPa at ambient temperature, and then the temperature to 950-980 K. Due to heating, the pressure increased to 61 ± 2 GPa. The sample was heated for four hours during which no changes were observed, except for a slight asymmetric broadening of the (220) reflection. Then the pressure was increased to 86 ± 2 GPa at a temperature of 970 ± 10 K (Figure 99) and after 5 to 6 hours of heating, all the reflections of ferropericlase became asymmetric and some showed splitting (Figure 100). A mixture of three phases (platinum, cubic and rhombohedral ferropericlases in the proportion 1:22:3 (Figure 100) had to be assumed to reproduce all features of the XRD pattern. On decompression, the reflections of ferropericlase remained split. The X-ray data of the quenched samples at ambient conditions showed the presence of two cubic phases with lattice parameters of 4.253(1) and 4.327(1) Å, respectively. The first corresponds to a ferropericlase with the composition 35 at.% FeO and 65 at.% MgO, and the second to an almost stoichiometric pure wüstite. Within the limit of error (~3%) no ferric iron was detected by Mössbauer spectroscopy in the ferropericlase before and after our experiments.
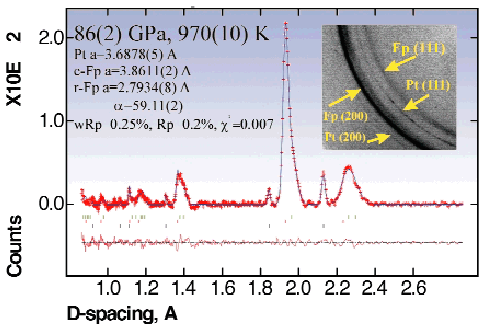 |
Fig. 100: Example of analysed X-ray spectrum collected at 970(10) K and 86(2) GPa after 6 hours of heating. It is possible to describe the diffraction pattern in terms of a mixture of three phases - platinum (lower marks), cubic and rhombohedral (r-Fp) ferropericlases (upper marks) which perfectly reproduce all features of the pattern. Inset shows example of images collected with monochromatic 0.3738 Å radiation with the FastScan imaging plate.
|
The X-ray studies of the externally heated samples in the diamond-anvil cell show that magnesiowüstite may dissociate into a magnesium- and a ferrous-rich oxide component. We cannot resolve the question about the size (width) of the immiscibility gap in the MgO-FeO system at high pressure or whether magnesiowüstite could dissociate completely into MgO and FeO after a sufficiently long time of heating. However, it is clear that magnesiowüstite could at least partially dissociate into a phase with lower density (magnesium rich phase, ~ 6.1 g/cm3) and a phase with higher density (iron rich, ~ 7.8 g/cm3) at 85 GPa, corresponding to a depth of 1900-2000 km (PREM). Such dissociation of magnesiowüstite may lead to the heterogeneity of the lower mantle.
References
[1] Y. Fei, and H.K. Mao, Science, 266, 1678 (1994).
[2] T.S. Duffy, R.J. Hemley, and H.K. Mao, Phys. Rev. Lett., 74, 1371 (1995).
Principal Publication and Authors
L.S. Dubrovinsky (a), N.A. Dubrovinskaia (a), S.K. Saxena, H. Annersten (a), E. Hålenius, H. Harryson, F. Tutti, S. Rekhi and T. Le Bihan (b), Science, 289, 430-432 (2000).
(a) Uppsala University (Sweden)
(b) ESRF



