- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Soft Condensed Matter
- Free and Lipid-covered Water and Gel Surfaces
Free and Lipid-covered Water and Gel Surfaces
The surface properties of gels are largely unexplored. We have studied phospholipid monolayers on the surface of aqueous clay gels, which, beside their fundamental interest, have also many industrial applications. Our idea was to investigate the free and lipid-covered surface of a liquid sol undergoing a sol-gel transition. Strong effects are expected on the monolayer structure since the 2-D system will now be coupled to a 3-D substrate of increasing viscoelasticity. We obtained for the first time detailed structural information on free sol and gel surfaces and on the 2-D ordering of phospholipid monolayers deposited on these phases.
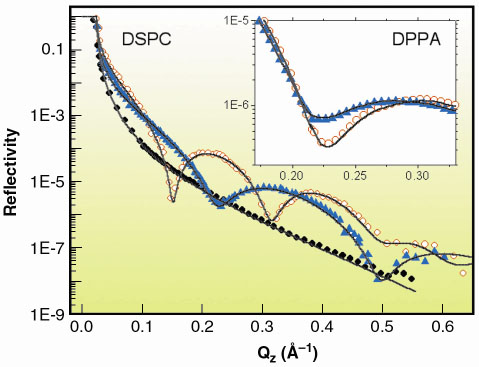 |
Fig. 56: X-ray reflectivity curves: ( |
The free surfaces of montmorillonite sol and gel phases were examined using X-ray specular reflectivity. The reflectivity profiles for water, sol and gel phases exhibit smooth structureless decays, as shown in Figure 56, and reveal that their surfaces are indistinguishable down to atomic length scales. The solid line is a fit to the data, assuming a simple refractive index step between air and the subphase smeared by a roughness of 3.4 Å. As the sol and gel surfaces exhibit a molecular flatness which is preserved through the sol-gel transition, a deposition of an amphiphilic lipid layer was attempted to examine the effect of "solidification" on the organisation of this bidimensional system. The experimental reflectivity curves for the DSPC phospholipid on water and on the gel phase are also shown in Figure 56. On both substrates, well-contrasted fringe patterns could be observed. The period of the fringes in the case of the gel is however shorter than in the case of water. The data indicate a single adsorption layer of mineral discs underneath the lipid headgroups that is induced by electrostatic interactions between the anionic silicate particles and the cationic tip of the DSPC headgroup (Figure 57). Electrostatic interactions induce a re-orientation of the lipid headgroup conformation and a reduction of the tilt angle of the lipid chains as indicated in Figure 57. This interpretation was confirmed by grazing incidence diffraction experiments.
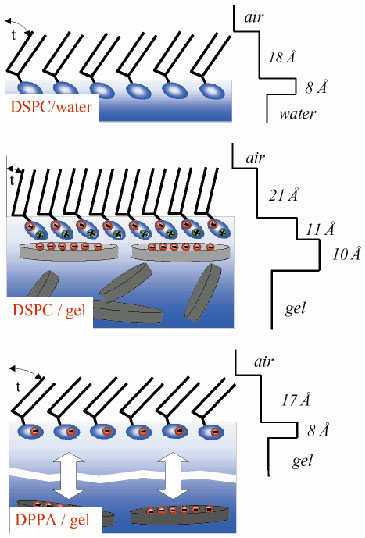 |
Fig. 57: Model for the organisation of phospholipid monolayers on water and gel substrates together with the corresponding density profiles: (Top) DSPC monolayer at the air/water interface, t is the tilt angle of the chains; (Middle) DSPC monolayer at the montmorillonite gel/air interface; (Bottom) DPPA monolayer at the montmorillonite sol-gel/air interface. |
Such an adsorption mechanism is backed by another series of experiments using another lipid, DPPA, bearing a negatively charged headgroup. In this case, no evidence for the formation of a surface layer of mineral discs can be extracted from the reflectivity curves (Figure 56, inset). Repulsive interactions between mineral particles and DPPA "push" the head groups toward the water surface causing an increase of tilt angle t of the chains (Figure 57).
Using X-ray surface techniques, we have shown that the free surface of a montmorillonite gel is of the same quality as a water surface. We have also demonstrated that the deposition of an appropriate amphiphilic monolayer on a montmorillonite gel surface induces the formation of a layer of mineral discs aligned parallel to the lipid-covered surface. This lipid/mineral double layer may act as a barrier for the permeation of various external species into the subphase but also makes the aqueous interface hydrophobic and sustained by a solid-like gel substrate.
Principal Publication and Authors:
B. Struth(a), F. Rieutord (b), O. Konovalov (a), G. Brezesinski (c), G. Grübel (a) and P. Terech (b), Phys. Rev. Lett., in print.
(a) ESRF
(b) UMR 5819 CEA-CNRS-Université J. Fourier, Grenoble (France)
(c) Max-Planck Institute of Colloids and Interfaces, Golm (Germany)



