- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Materials
- Absence of the High-pressure CsCl Phase in InAs
Absence of the High-pressure CsCl Phase in InAs
One of the common properties of covalent semiconductors is that all of them present first order phase transitions under pressure that transform their open four-fold coordinated structures to more dense six-fold coordinated structures. The higher density structures to which tetrahedrally coordinated compounds were thought to transform are structures that exist at ambient pressure in more ionic A(n)B(8-n) octet compounds. For example, III-V zincblende (ZB) semiconductors were expected to transform into the NaCl phase and eventually to the CsCl phase [1].
However, recent theoretical work [2] predicts that, while statically stable, the CsCl phase is dynamically unstable at high pressures for the more ionic III-V semiconductors. By analysing the destabilising transverse acoustic modes of CsCl the authors were able to identify two candidate structures, InBi (P4/nmm) and AuCd (Pmma) that could possibly replace CsCl. Experimental examination of these predictions was obviously required.
We have examined the InAs compound, because it is among the more ionic III-V semiconductors and has the lowest predicted transition pressure to CsCl.
No direct investigation of the pressure evolution of the local structure using X-ray absorption spectroscopy (XAS) has, to our knowledge, been reported on InAs. However, XAS can play an important complementary role in identifying the local structure and the degree of short range chemical (site) ordering in new phases, since it probes selectively the local environment of the absorber atom and is sensitive to the surrounding neighbours. The high pressure XAS and XRD measurements were carried out on beamlines ID24 and ID30 respectively.
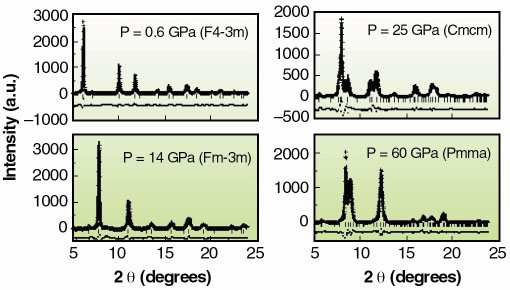 |
Fig. 124: XRD profiles, Rietveld refinements and residuals at 0.6 GPa (F-43m), 14 GPa (Fm3m), 25 GPa (Cmcm) and at 60 GPa (Pmma). |
Figure 124 reports XRD integrated profiles: the observed structural sequence is the following: F-43m Fm3m
Cmcm
Pmma, with transition pressures 7.5(5) GPa, 15(1) GPa and 30(3) GPa. While we are unable to address the long range site ordering issue from our high pressure XRD data, we can yield valuable short range chemical order/disorder (sr-co / sr-cd) information using our XANES data (Figure 125a). We compared the latter to full multiple scattering calculations using a self-consistent energy dependent exchange correlation Hedin Lundqvist potential. The variations as a function of pressure in the features of the XAS spectra (Figure 125b) can be associated to the following structural sequence:
F-43m sr-co Fm3m
sr-co Cmcm
sr-cd Pmma.
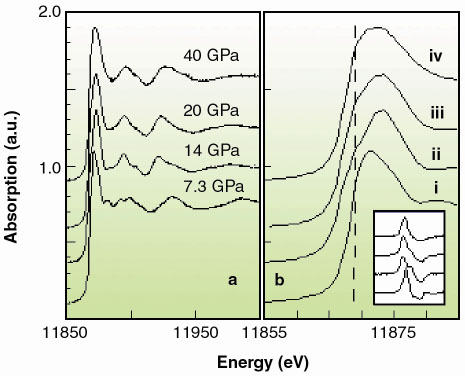 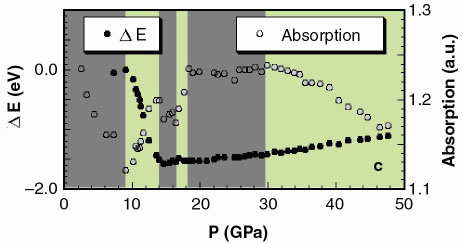 |
Fig. 125: a) XAS spectra and b) edge region at i) 7.3, ii) 14, iii) 20, and iv) 40 GPa. The inset shows the trend on the derivatives. c) energy shift of the absorption onset, defined as the position of the maximum of the first derivative at the edge (full dots) and absorption at 11869.1 eV (empty dots). Dark and light areas correspond respectively to single phase (Zincblende, NaCl, Cmcm) and phase coexistence regions (Zincblende-NaCl, NaCl-Cmcm, Cmcm-Pmma). |
In conclusion, using X-ray diffraction and absorption techniques, we have demonstrated that InAs does not transform to the CsCl phase as previously thought, but to a short range chemically-disordered Pmma phase, in agreement with recent phonon dynamics calculations. This finding has important implications for the structural systematics of semiconductors. InAs is the first of the III-V semiconductors for which existence of a theoretically predicted phase is verified a posteriori. This leave the experimental examination of the remaining systems, foreseen to have a similar behaviour, InP, GaP and GaAs, to be undertaken.
References
[1] J.C. Phillips, Bonds and Bands in Semiconductors, Academic Press, New York, p. 200 (1973).
[2] K. Kim, V. Ozolins and A. Zunger, Phys. Rev. B 60, R8449 (1999).
Principal Publication and Authors
S. Pascarelli (a), G. Aquilanti (a), W. Crichton (a), T. Le Bihan (a), M. Mezouar (a), S. De Panfilis (b), J.P. Itié (c) and A. Polian (c), submitted.
(a) ESRF
(b) Unita di Ricerca INFM and Dip. di Fisica, Universitá di Camerino (Italy)
(c) Physique des Milieux Condensés, C.N.R.S.,Université Pierre et Marie Curie, Paris (France)



