Soils & sediments
Speciation and microspatial patterns of nutrients and pollutants in soils and sediments
Soils have important ecological functions, like food production, environmental filter and acts as a potential carbon storage/source. Thus, it is of pivotal importance to understand the changes of speciation and micro-spatial patterns of nutrients and pollutants in soils. The microscopes at ID21 have unraveled the cycling of nutrients like P, S and Ca in soils, and their potential role in carbon sequestration. On the other hand, researchers and users of ID21 have also monitored the transference, and change of pollutants from soils to plants.
Sulphur
 |
J. Prietzel, J. Thieme, U. Neuhäusler, J. Susini and I. Kogel-Knaber, & Miliani, C. "Speciation of sulphur in soils and soil particles by X-ray spectromicroscopy". European Journal of Soil Science, 54, 423-433 (2003). |
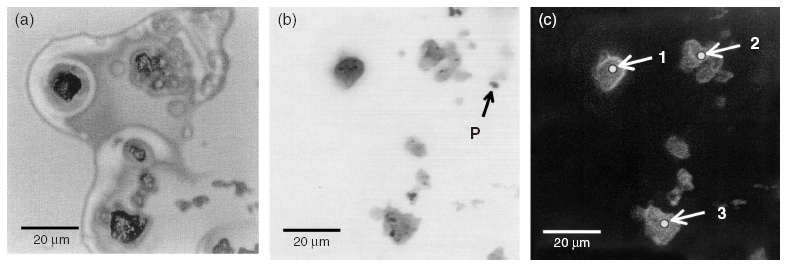
Current wet chemical methods for the speciation of sulphur (S) in soils are inaccurate and do not allow one to assess the S speciation of individual soil particles and colloids. X-ray microscopy and Near Edge X-ray Absorption Fine structure Spectroscopy (NEXAFS) can be used to study individual species of S at the K-adsorption edge. We have used these techniques to identify and quantify S species in bulk soil, soil particles and colloids from Oh and Bh horizons of two forested Podzols. The partitioning of soil sulphur as determined on bulk samples of the Oh horizons by X-ray spectromicroscopy agreed fairly well with the results of a conventional S speciation for the soil at Schluchsee, and reasonably well for that at Rotherdbach. The NEXAFS analyses on individual soil particles revealed that they are richer in reduced organic sulphur than the bulk soil for the Schluchsee Oh and richer in sulphate for Rotherdbach Oh. The techniques can be used reliably to separate and quantify sulphur species with different oxidation states in the soil. The combination of X-ray transmission and sulphur fluorescence images with unfocused and focused NEXAFS spectra at the K-adsorption edge of sulphur at specific microsites allowed us to compare the distribution of S species in bulk soil with that of distinct soil particles and soil colloids. Moreover, we can use it to assess the spatial distribution of different S species on soil particles on a scale of a few hundred nanometres.
Iron
 |
T. Rennert, K. Eusterhues, V. De Andrade and K. U. Totsche, "Iron species in soils on a mofette site studied by Fe K-edge X-ray absorption near-edge spectroscopy", Chemical Geology, 332–333, 116-123 (2012). |
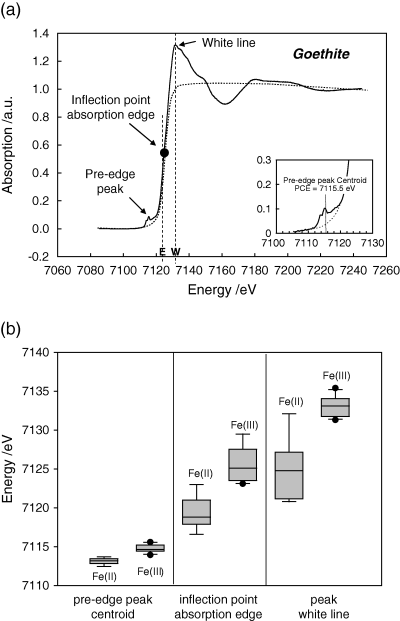
Iron speciation in soils is still poorly understood. We have investigated inorganic and organic standard substances, diluted mixtures of common Fe minerals in soils (pyrite, ferrihydrite, goethite), soils in a forested watershed which constitute a toposequence with a hydrological gradient (Dystric Cambisol, Dystric Planosol, Rheic Histosol), and microsites of a dissected soil aggregate by X-ray Absorption Near Edge Spectroscopy (XANES) at the iron K-edge (7112 eV) to identify different Fe(II) and Fe(III) components. We calculated the pre-edge peak centroid energy of all spectra and quantified the contribution of different organic and inorganic Fe-bearing compounds by Linear Combination Fitting (LCF) conducted on the entire spectrum (E = 7085–7240 eV) and on the pre-edge peak. Fe-XANES conducted on organic and inorganic standards and on synthetic mixtures of pyrite, ferrihydrite and goethite showed that by calculating the pre-edge peak centroid energy, the Fe(II)/Fe(III) ratio of different Fe-bearing minerals (Fe sulphides, Fe oxyhydroxides) in mineral mixtures and soils can be quantified with reasonable accuracy. A more accurate quantification of the Fe(II)/Fe(III) ratio was possible with LCF conducted on the entire XANES spectrum. For the soil toposequence, an increased groundwater influence from the Cambisol to the Histosol was reflected in a larger contribution of Fe(II) compounds (Fe(II) silicate, Fe monosulphide, pyrite) and a smaller contribution of Fe(III) oxyhydroxides (ferrihydrite, goethite) to total iron both in the topsoil and the subsoil. In the organic topsoils, organically bonded Fe (33–45% of total Fe) was 100% Fe(III). For different microsites in the dissected aggregate, spatial resolution ofμ-XANES revealed different proportions of Fe(II) and Fe(III) compounds. Fe K-edge XANES andμ-XANES allows an approximate quantification of Fe(II) and Fe(III) and different Fe compounds in soils and (sub)micron regions of soil sections, such as mottles, concretions, and rhizosphere regions, thus opening new perspectives in soil research.
 |
J. Prietzel, N. Tyufekchieva, K. Eusterhues, I. Kögel-Knabner, J. Thieme, D. Paterson, I. McNulty, M. de Jonge, D. Eichert and M. Salomé, "Anoxic versus oxic sample pretreatment: Effects on the speciation of sulfur and iron in well-aerated and wetland soils as assessed by X-ray absorption near-edge spectroscopy (XANES)", Geoderma, 153, 318-330 (2009). |
For a toposequence with increasing groundwater influence (Cambisol, Stagnosol, Histosol) and with different groundwater regimes (Histosols 1 and 2) in a forested watershed in the Fichtelgebirge (Germany), the speciation of sulfur (S) and iron (Fe) in the soils was assessed by X-ray absorption near-edge spectroscopy (XANES) after anoxic and conventional oxic sample pretreatments. For samples with anoxic pretreatment, the contribution of reduced inorganic S compounds (monosulfide, pyrite) to total S increased with soil depth for the Cambisol and the Stagnosol, but decreased for the Histosols; the opposite trend was noticed for the contribution of reduced organic S (organic mono- and disulfides, thiols). The contribution of reduced S to the soil S pool increased and the contribution of oxidized S compounds decreased in the sequence Cambisol–Stagnosol–Histosol 1 (permanently anoxic). Histosol 2 (seasonally oxic) showed a markedly larger contribution of oxidized and intermediate S compounds to total S than Histosol 1. The dominating Fe-bearing phases in the Cambisol were Fe(III) oxyhydroxides; the contribution of sulfide-bound Fe was < 5% of total Fe in all horizons. In Histosol 1, the contribution of sulfide-bound Fe increased with soil depth up to 50% in the Cr horizon, whereas in Histosol 2 Fe(III) phases strongly dominated in all horizons. After conventional oxic sample pretreatment, the contribution of reduced inorganic S to total S was markedly decreased in all soils. In the organic surface horizons, the contribution of reduced organic S was increased to the same extent; the contribution of oxidized S (sulfate) remained more or less unchanged. In the mineral soil, the contribution of sulfate and the mean oxidation state of sulfur (MOS) were strongly increased after oxic sample preparation. In Histosol 1, oxic sample pretreatment resulted in oxidation of labile Fe(II) compounds, probably sulfides or Fe(II)–S-org-complexes, to Fe(III). Our study shows that for anoxic wetland soils which contain inorganic sulfide and/or divalent Fe, the exclusion of O2 during the entire period between sampling and analysis is crucial for a correct S and Fe speciation. Only after appropriate sample preparation, clear relationships between the mean oxidation states of S and Fe (MOFe) on one hand and soil hydrological conditions on the other become evident: a concomitant systematic decrease of MOS and MOFe from the well-aerated Cambisol to the permanently anoxic Histosol 1, and larger MOS and MOFe in the seasonally oxic Histosol 2 than in Histosol 1 indicate a close coupling of S and Fe cycling in the soils. Finally, the results of our study suggest that in organic horizons of wetland soils inorganic sulfide S is overestimated and reduced organic S is underestimated by S K-edge XANES, if a significant portion of the thiol groups in reduced organic S is complex-bound to Fe2+ or other chalcophilic metal cations. This is supported by the observation that synthetic organic compounds (cysteine; 1,3,5-trimer-captotriazine [TMT]; ferredoxin) after addition of Fe show spectra with pre-edge peaks at energies < 2472 eV that are typical for inorganic sulfide.
 |
J. Prietzel, J. Thieme, K. Eusterhues and D. Eichert, "Iron speciation in soils and soil aggregates by synchrotron-based X-ray microspectroscopy (XANES, µ-XANES)", European Journal of Soil Science, 58, 1027-1041 (2007). |
Iron speciation in soils is still poorly understood. We have investigated inorganic and organic standard substances, diluted mixtures of common Fe minerals in soils (pyrite, ferrihydrite, goethite), soils in a forested watershed which constitute a toposequence with a hydrological gradient (Dystric Cambisol, Dystric Planosol, Rheic Histosol), and microsites of a dissected soil aggregate by X-ray Absorption Near Edge Spectroscopy (XANES) at the iron K-edge (7112 eV) to identify different Fe(II) and Fe(III) components. We calculated the pre-edge peak centroid energy of all spectra and quantified the contribution of different organic and inorganic Fe-bearing compounds by Linear Combination Fitting (LCF) conducted on the entire spectrum (E = 7085–7240 eV) and on the pre-edge peak. Fe-XANES conducted on organic and inorganic standards and on synthetic mixtures of pyrite, ferrihydrite and goethite showed that by calculating the pre-edge peak centroid energy, the Fe(II)/Fe(III) ratio of different Fe-bearing minerals (Fe sulphides, Fe oxyhydroxides) in mineral mixtures and soils can be quantified with reasonable accuracy. A more accurate quantification of the Fe(II)/Fe(III) ratio was possible with LCF conducted on the entire XANES spectrum. For the soil toposequence, an increased groundwater influence from the Cambisol to the Histosol was reflected in a larger contribution of Fe(II) compounds (Fe(II) silicate, Fe monosulphide, pyrite) and a smaller contribution of Fe(III) oxyhydroxides (ferrihydrite, goethite) to total iron both in the topsoil and the subsoil. In the organic topsoils, organically bonded Fe (33–45% of total Fe) was 100% Fe(III). For different microsites in the dissected aggregate, spatial resolution ofμ-XANES revealed different proportions of Fe(II) and Fe(III) compounds. Fe K-edge XANES andμ-XANES allows an approximate quantification of Fe(II) and Fe(III) and different Fe compounds in soils and (sub)micron regions of soil sections, such as mottles, concretions, and rhizosphere regions, thus opening new perspectives in soil research.
|
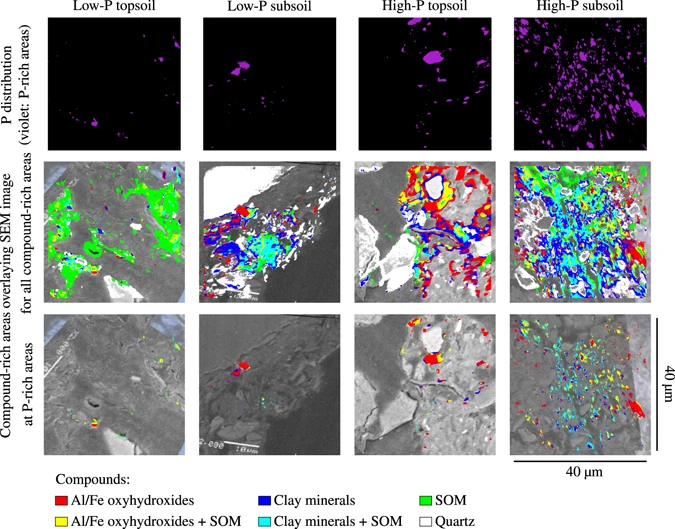
F. Werner, C. W. Mueller, J. Thieme, A. Gianoncelli, C. Rivard, C. Höschen and J. Prietzel, "Micro-scale heterogeneity of soil phosphorus depends on soil substrate and depth", Scientific Reports, 7, 3203 (2017). |
|
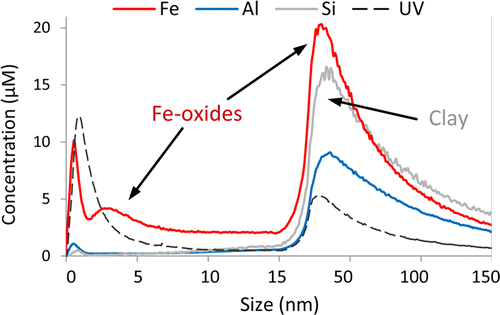
Soils comprise various heterogeneously distributed pools of lithogenic, free organic, occluded, adsorbed, and precipitated phosphorus (P) forms, which differ depending on soil forming factors. Small-scale heterogeneity of element distributions recently has received increased attention in soil science due to its influence on soil functions and soil fertility. We investigated the micro-scale distribution of total P and different specific P binding forms in aggregates taken from a high-P clay-rich soil and a low-P sandy soil by combining advanced spectrometric and spectroscopic techniques to introduce new insights on P accessibility and availability in soils. Here we show that soil substrate and soil depth determine micro-scale P heterogeneity in soil aggregates. In P-rich areas of all investigated soil aggregates, P was predominantly co-located with aluminium and iron oxides and hydroxides, which are known to strongly adsorb P. Clay minerals were co-located with P only to a lesser extent. In the low-P topsoil aggregate, the majority of the P was bound organically. Aluminium and iron phosphate predominated in the quartz-rich low-P subsoil aggregate. Sorbed and mineral P phases determined P speciation in the high-P top- and subsoil, and apatite was only detected in the high-P subsoil aggregate. Our results indicate that micro-scale spatial and chemical heterogeneity of P influences P accessibility and bioavailability.
|
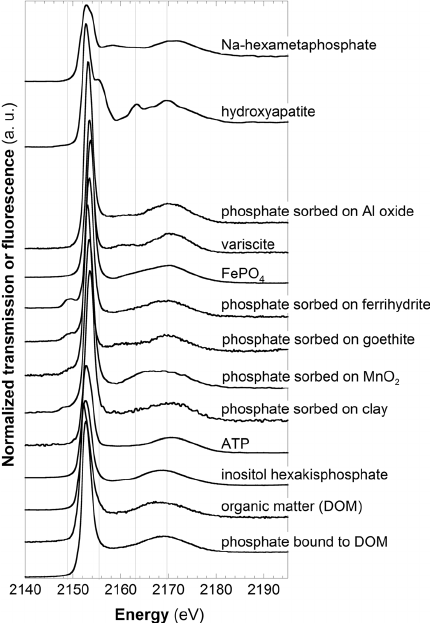
C. Rivard, B. Lanson and M. Cotte, "Phosphorus speciation and micro-scale spatial distribution in North-American temperate agricultural soils from micro X-ray fluorescence and X-ray absorption near-edge spectroscopy", Plant and Soil, 401, 7-22 (2016). |
|
Phosphorus (P) is an essential nutrient for plants but its low availability often necessitates amendments for agronomical issues. Objectives were to determine P spatial distribution and speciation that remain poorly understood in cultivated soils. Aquic Argiudoll soil samples developed on a calcareous loam glacial till were collected from experimental plots submitted to contrasting crop rotations and amendments. Micro-X-ray fluorescence (μ-XRF) maps were collected on undisturbed samples. X-ray absorption near edge structure (XANES) spectra were collected on bulk samples and on fractions thereof, and on points of interests selected from μ-XRF maps. Results were compared with chemical analyses and extraction techniques results. Chemical analyses show variations in total and exchangeable P contents depending on the samples but no significant difference is observed in terms of P distribution and speciation. P distribution is dominated by a low-concentration diffuse background with a minor contribution from minute hot spots. P speciation is dominated by phosphate groups bound to clay-humic complexes. No modification of P distribution and speciation is observed close to roots. This study evidenced minor effect of cropping and fertilizing practices on P speciation in cultivated soils. Despite analytical challenges, the combined use of μ-XRF and XANES provides relevant information on P speciation in heterogeneous soil media.
|
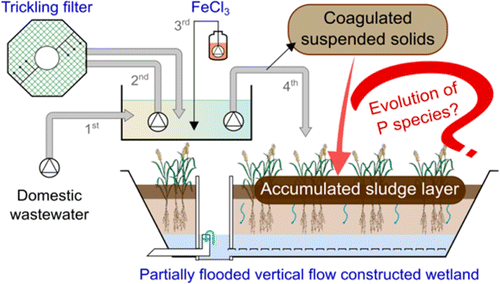
B. Kim, M. Gautier, C. Rivard, C. Sanglar, P. Michel and R. Gourdon, "Effect of Aging on Phosphorus Speciation in Surface Deposit of a Vertical Flow Constructed Wetland", Environmental science & technology, 49, 4903-4910 (2015). |
|
This study was conducted to determine phosphorus (P) species captured in a vertical-flow constructed wetland (VFCW) system combining a trickling filter followed by FeCl3 injection for phosphate coagulation. Suspended solids (SS) thus formed accumulated over time at the VFCW surface and transformed into a sludge deposit layer, which was shown to concentrate most of the P captured in the system. In order to investigate the effect of aging on P species, representative SS and sludge samples were taken from a wastewater treatment plant that had been in operation for 8 years and analyzed using P fractionation, solution 31P NMR spectroscopy, and P and Fe K-edge XANES spectroscopy. A partial mineralization of organic matter was shown by comparing organic carbon contents of SS and sludge materials. Chemical fractionations combined with P and Fe K-edge XANES spectroscopy showed that P was predominantly bound to iron within both samples in the form of ferric phosphate, rather than adsorbed onto ferric oxyhydroxide. Calcium-bound P was more significantly observed in sludge than in SS, suggesting that aging induced the recombination of part of the organic and iron-bound P species into calcium-bound forms, as a possible consequence of the partial mineralization of organic matter.
Phosphorus
|
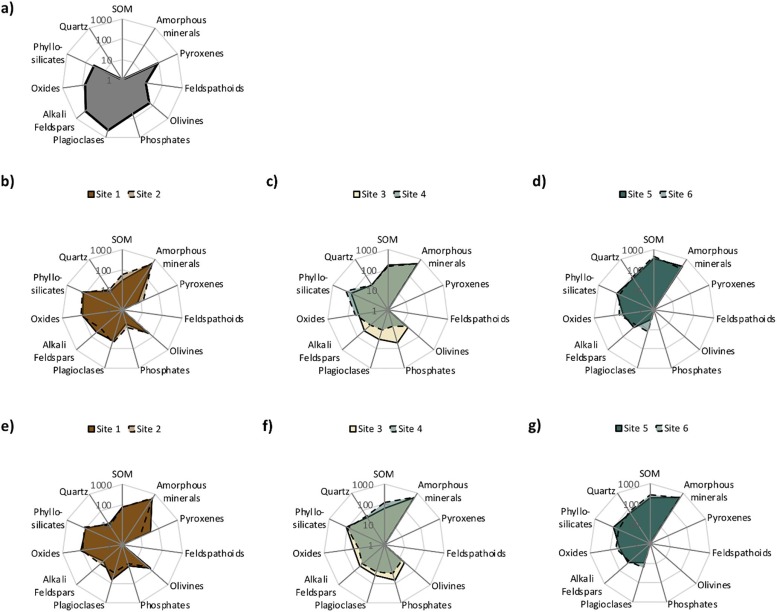
C. Vogel, J. Helfenstein, M. S. Massey, R. Sekine, R. Kretzschmar, L. Beiping, T. Peter, O. A. Chadwick, F. Tamburini, C. Rivard, H. Herzel, C. Adam, A. E. Pradas del Real, H. Castillo-Michel, L. Zuin, D. Wang, R. Félix, B. Lassalle-Kaiser and E. Frossard, "Microspectroscopy reveals dust-derived apatite grains in acidic, highly-weathered Hawaiian soils", Geoderma, 381, 114681 (2021). |
|
Dust deposition is an important source of phosphorus (P) to many ecosystems. However, there is little evidence of dust-derived P-containing minerals in soils. Here we studied P forms along a well-described climatic gradient on Hawaii, which is also a dust deposition gradient. Soil mineralogy and soil P forms from six sites along the climatic gradient were analyzed with bulk (X-ray diffraction and P K-edge X-ray absorption near edge structure) and microscale (X-ray fluorescence, P K-edge X-ray absorption near edge structure, and Raman) analysis methods. In the wettest soils, apatite grains ranging from 5 to 30 µm in size were co-located at the micro-scale with quartz, a known continental dust indicator suggesting recent atmospheric deposition. In addition to co-location with quartz, further evidence of dust-derived P included backward trajectory modeling indicating that dust particles could be brought to Hawaii from the major global dust-loading areas in central Asia and northern Africa. Although it is not certain whether the individual observed apatite grains were derived from long-distance transport of dust, or from local dust sources such as volcanic ash or windblown fertilizer, these observations offer direct evidence that P-containing minerals have reached surface layers of highly-weathered grassland soils through atmospheric deposition.
|
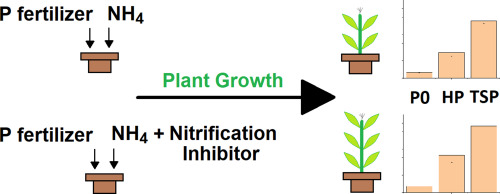
C. Vogel, R. Sekine, J. Huang, D. Steckenmesser, D. Steffens, T. Huthwelker, C. N. Borca, A. E. P. del Real, H. Castillo-Michel and C. Adam, "Effects of a nitrification inhibitor on nitrogen species in the soil and the yield and phosphorus uptake of maize", Science of the Total Environment", Analytical Chemistry, 715, 136895 (2020). |
|
Phosphorus (P) resource availability is declining and the efficiency of applied nutrients in agricultural soils is becoming increasingly important. This is especially true for P fertilizers from recycled materials, which often have low plant availability. Specific co-fertilization with ammonium can enhance P plant availability in soils amended with these P fertilizers, and thus the yield of plants. To investigate this effect, we performed a pot experiment with maize in slightly acidic soil (pH 6.9) with one water-soluble (triple superphosphate [TSP]) and two water-insoluble (sewage sludge-based and hyperphosphate [Hyp]) P fertilizers and an ammonium sulfate nitrate with or without a nitrification inhibitor (NI). The dry matter yield of maize was significantly increased by the NI with the Hyp (from 14.7 to 21.5 g/pot) and TSP (from 40.0 to 45.4 g/pot) treatments. Furthermore, P uptake was slightly increased in all three P treatments with the NI, but not significantly. Olsen-P extraction and P K-edge micro-X-ray absorption near-edge structure (XANES) spectroscopy showed that apatite-P of the water-insoluble P fertilizers mobilized during the plant growth period. In addition, novel nitrogen (N) K-edge micro-XANES spectroscopy and the Mogilevkina method showed that the application of an NI increased the fixation of ammonium in detectable hot spots in the soil. Thus, the delay in the nitrification process by the NI and the possible slow-release of temporarily fixed ammonium in the soil resulted in a high amount of plant available ammonium in the soil solution. This development probably decreases the rhizosphere pH due to release of H+ by plants during ammonium uptake, which mobilizes phosphorus in the amended soil and increases the dry matter yield of maize. This is especially important for water-insoluble apatite-based P fertilizers (conventional and recycled), which tend to have poor plant availability.
|
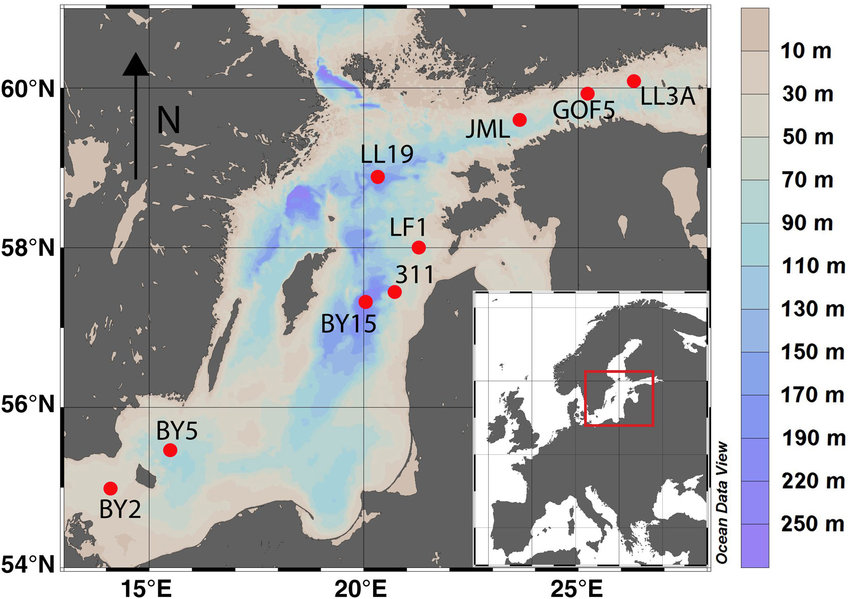
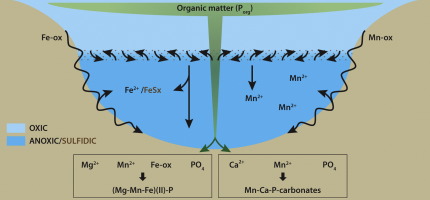
M. Hermans, W. K. Lenstra, N. A. G. M. van Helmond, T. Behrends, M. Egger, M. J. M. Séguret, E. Gustafsson, B. G. Gustafsson and C. P. Slomp, "Impact of natural re-oxygenation on the sediment dynamics of manganese, iron and phosphorus in a euxinic Baltic Sea basin", Geochimica et Cosmochimica Acta, 246, 174-196 (2019). |
|
The Baltic Sea is characterized by the largest area of hypoxic (oxygen (O2) < 2 mg L−1) bottom waters in the world’s ocean induced by human activities. Natural ventilation of these O2-depleted waters largely depends on episodic Major Baltic Inflows from the adjacent North Sea. In 2014 and 2015, two such inflows led to a strong rise in O2 and decline in phosphate (HPO42−) in waters below 125 m depth in the Eastern Gotland Basin. This provided the opportunity to assess the impact of such re-oxygenation events on the cycles of manganese (Mn), iron (Fe) and phosphorus (P) in the sediment for the first time. We demonstrate that the re-oxygenation induced the activity of sulphur (S)-oxidising bacteria, known as Beggiatoaceae in the surface sediment where a thin oxic and suboxic layer developed. At the two deepest sites, strong enrichments of total Mn and to a lesser extent Fe oxides and P were observed in this surface layer. A combination of sequential sediment extractions and synchrotron-based X-ray spectroscopy revealed evidence for the abundant presence of P-bearing rhodochrosite and Mn(II) phosphates. In contrast to what is typically assumed, the formation of Fe oxides in the surface sediment was limited. We attribute this lack of Fe oxide formation to the high flux of reductants, such as sulphide, from deeper sediments which allows Fe(II) in the form of FeS to be preserved and restricts the penetration of O2 into the sediment. We estimate that enhanced P sequestration in surface sediments accounts for only ∼5% of water column HPO42− removal in the Eastern Gotland Basin linked to the recent inflows. The remaining HPO42− was transported to adjacent areas in the Baltic Sea. Our results highlight that the benthic O2 demand arising from the accumulation of organic-rich sediments over several decades, the legacy of hypoxia, has major implications for the biogeochemical response of euxinic basins to re-oxygenation. In particular, P sequestration in the sediment in association with Fe oxides is limited. This implies that artificial ventilation projects that aim at removing water column HPO42− and thereby improving water quality in the Baltic Sea will likely not have the desired effect.
|
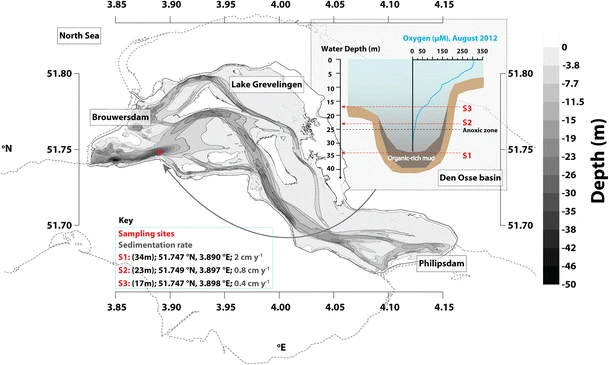
F. Sulu-Gambari, M. Hagens, T. Behrends, D. Seitaj, F. J. Meysman, J. Middelburg and C. P. Slomp, "Phosphorus cycling and burial in sediments of a seasonally hypoxic marine basin", Estuaries and coasts, 41, 921-939 (2018). |
|
Recycling of phosphorus (P) from sediments contributes to the development of bottom-water hypoxia in many coastal systems. Here, we present results of a year-long assessment of P dynamics in sediments of a seasonally hypoxic coastal marine basin (Lake Grevelingen, the Netherlands) in 2012. Sequential phosphorus extractions (SEDEX) and X-ray absorption spectroscopy (XAS) indicate that P was adsorbed to Fe-(III)-(oxyhydr)oxides when cable bacteria were active in the surface sediments in spring. With the onset of summer hypoxia, sulphide-induced dissolution of the Fe-(III)-(oxyhydr)oxides led to P release to the pore water and overlying water. The similarity in authigenic Ca-P concentrations in the sediment and suspended matter suggest that Ca-P is not formed in situ. The P burial efficiency was ≤ 32%. Hypoxia-driven sedimentary P recycling had a major impact on the water-column chemistry in the basin in 2012. Water-column monitoring data indicate up to ninefold higher surface water concentrations of phosphate in the basin in the late 1970s and a stronger hypoxia-driven seasonal P release from the sediment. The amplified release of P from the sediment in the past is attributed to the presence of a larger pool of Fe-bound P in the basin prior to the first onset of hypoxia. Given that P is not limiting, primary production in the basin has not been affected by the decadal changes in P availability and recycling over the past 40 years. The changes in P dynamics on decadal time scales were not recorded in sediment profiles of total P or organic C/total P.
|
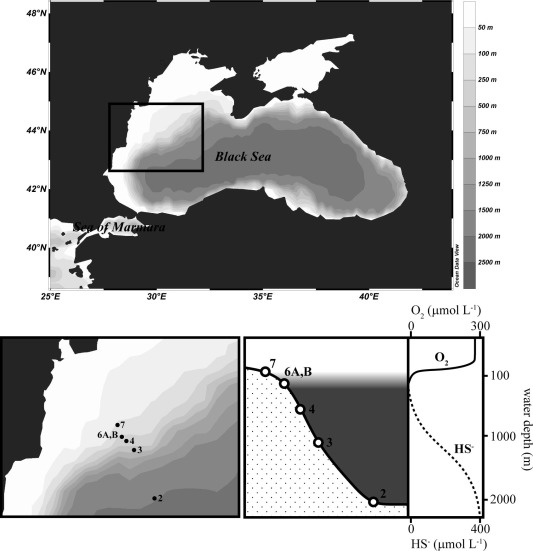
P. Kraal, N. Dijkstra, T. Behrends and C. P. Slomp, "Phosphorus burial in sediments of the sulfidic deep Black Sea: Key roles for adsorption by calcium carbonate and apatite authigenesis", Geochimica et Cosmochimica Acta, 204, 140-158 (2017). |
|
Sedimentary burial of the essential nutrient phosphorus (P) under anoxic and sulfidic conditions is incompletely understood. Here, we use chemical and micro-scale spectroscopic methods to characterize sedimentary P burial along a water column redox transect (six stations, 78–2107 m water depth) in the Black Sea from the shelf with its oxygenated waters to the anoxic and sulfidic deep basin. Organic P is an important P pool under all redox regimes, accounting for up to 60% of P burial. We find a general down-core increase in the relative importance of organic P, especially on the shelf where P bound to iron (Fe) and manganese (Mn) (oxyhydr)oxides is abundant in the uppermost sediment but rapidly declines in concentration with sediment depth. Our chemical and spectroscopic data indicate that the carbonate-rich sediments (Unit I, ∼3000 years, ∼0–30 cm depth) of the sulfidic deep Black Sea contain three major P pools: calcium phosphate (apatite), organic P and P that is strongly associated with CaCO3 and possibly clay surfaces. Apatite concentrations increase from 5% to 25% of total P in the uppermost centimeters of the deep basin sediments, highlighting the importance of apatite formation for long-term P burial. Iron(II)-associated P (ludlamite) was detected with X-ray absorption spectroscopy but was shown to be a minor P pool (∼5%), indicating that lateral Fe–P transport from the shelf (“shuttling”) likely occurs but does not impact the P burial budget of the deep Black Sea. The CaCO3–P pool was relatively constant throughout the Unit I sediment interval and accounted for up to 55% of total P. Our results highlight that carbonate-bound P can be an important sink for P in CaCO3-rich sediments of anoxic, sulfidic basins and should also be considered as a potential P sink (and P source in case of CaCO3 dissolution) when reconstructing past ocean P dynamics from geological records.
|
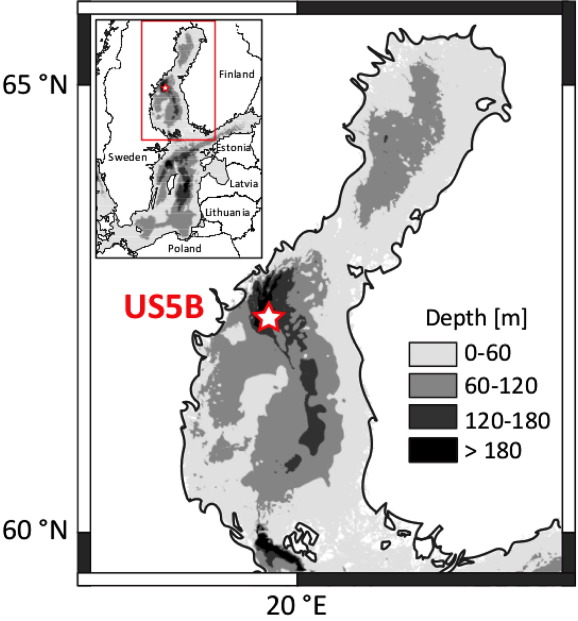
N. Dijkstra, C. P. Slomp and T. Behrends, "Vivianite is a key sink for phosphorus in sediments of the Landsort Deep, an intermittently anoxic deep basin in the Baltic Sea", Chemical Geology, 438, 58-72 (2016). |
|
Phosphorus (P) is an essential nutrient for marine organisms. Its burial in hypoxic and anoxic marine basins is still incompletely understood. Recent studies suggest that P can be sequestered in sediments of such basins as reduced iron (Fe)-P but the exact phase and the underlying mechanisms that lead to its formation are unknown. In this study, we investigated sediments from the deepest basin in the Baltic Sea, the Landsort Deep (site M0063), that were retrieved during the Integrated Ocean Drilling Project (IODP) Baltic Sea Paleoenvironment Expedition 347. The record comprises the whole brackish/marine Littorina Sea stage including past intervals of extensive hypoxia in the Baltic Sea that occurred during the Holocene Thermal Maximum (HTMHI) and the Medieval Climate Anomaly (MCA1HI and MCA2HI). Various redox proxies (e.g. the presence of laminations and high Mo contents) suggest almost permanent bottom water hypoxia during the Littorina Sea stage in the Landsort Deep. The bottom waters were likely even seasonally anoxic or sulfidic during the MCA1HI and MCA2HI, and permanently sulfidic during the HTMHI. With the use of micro-analysis of sieved minerals (SEM-EDS, XRD and synchrotron-based XAS), we show that Mn- and Mg-rich vivianite crystals are present at various depths in the Littorina Sea sediments. We also have indications for vivianite in the MCA1HI, MCA2HI and HTMHI deposits. The formation of vivianite thus likely explains the high Fe-bound P fraction throughout the whole Littorina Sea stage. Shuttling of Fe and Mn from the shelves into the basin and high inputs of P in settling organic matter are likely key drivers for vivianite formation. Our study shows that vivianite can likely form in near-surface sediments under a broad range of bottom water redox conditions, varying from hypoxic and anoxic to sulfidic.
|
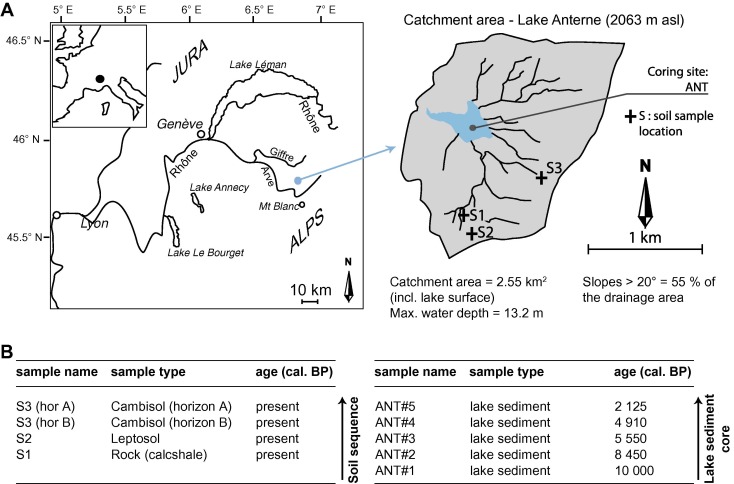
C. Giguet-Covex, J. Poulenard, E. Chalmin, F. Arnaud, C. Rivard, J. P. Jenny and J. M. Dorioz, "XANES spectroscopy as a tool to trace phosphorus transformation during soil genesis and mountain ecosystem development from lake sediments", Geochimica et Cosmochimica Acta & technology, 118, 129-147 (2013). |
|
The aim of this study is to investigate phosphorus (P) species modifications triggered by soil genesis and mountain ecosystem development after glacial retreat using a lake sediment archive (Lake Anterne, North French Alps). Five lake sediment samples, representative of different stages of soil and ecosystem development, were selected for P speciation analyses. Furthermore, a sequence of current soils from the catchment was analyzed to better constrain our interpretations of the lacustrine archive. Synchrotron techniques (X-ray Fluorescence (XRF) mapping and P K-edge X-ray absorption near edge structure (XANES) spectroscopy) were applied to lake sediments, soils, and standards (mineral and organic) to distinguish between different P species. The results show that soil development during the first millennia of the Holocene triggered increased P species diversity. At the onset of the Holocene, P was present as apatite when rocks and leptosols dominated the catchment. Pedogenic processes then led to apatite dissolution and the formation of large amounts of P on metal/clay-organic complexes. P geochemistry during the main step of soil genesis (early leptosols dominated by apatite, low weathered cambisols with P mainly adsorbed on iron oxides, highly weathered podzols with large amounts of P on Al/Fe/clay organic complexes) is thus clearly recorded in lake sediments. P K-edge XANES spectroscopy is particularly relevant as qualitative method to study P species in soils and lake sediments at high spatial resolution. Such resolution is needed to reveal the diversity of small P particles and like this better characterize the P cycle and improve our understanding of ecosystem evolution.
Sediments
|
M. Egger, T. Jilbert, T. Behrends, C. Rivard and C. P. Slomp, "Vivianite is a major sink for phosphorus in methanogenic coastal surface sediments", Geochimica et Cosmochimica Acta , 169, 217-235 (2015). |
|
Studies of authigenic phosphorus (P) minerals in marine sediments typically focus on authigenic carbonate fluorapatite, which is considered to be the major sink for P in marine sediments and can easily be semi-quantitatively extracted with the SEDEX sequential extraction method. The role of other potentially important authigenic P phases, such as the reduced iron (Fe) phosphate mineral vivianite (Fe(II)3(PO4)*8H2O) has so far largely been ignored in marine systems. This is, in part, likely due to the fact that the SEDEX method does not distinguish between vivianite and P associated with Fe-oxides. Here, we show that vivianite can be quantified in marine sediments by combining the SEDEX method with microscopic and spectroscopic techniques such as micro X-ray fluorescence (μXRF) elemental mapping of resin-embedded sediments, as well as scanning electron microscope–energy dispersive spectroscopy (SEM–EDS) and powder X-ray diffraction (XRD). We further demonstrate that resin embedding of vertically intact sediment sub-cores enables the use of synchrotron-based microanalysis (X-ray absorption near-edge structure (XANES) spectroscopy) to differentiate between different P burial phases in aquatic sediments. Our results reveal that vivianite represents a major burial sink for P below a shallow sulfate/methane transition zone in Bothnian Sea sediments, accounting for 40–50% of total P burial. We further show that anaerobic oxidation of methane (AOM) drives a sink-switching from Fe-oxide bound P to vivianite by driving the release of both phosphate (AOM with sulfate and Fe-oxides) and ferrous Fe (AOM with Fe-oxides) to the pore water allowing supersaturation with respect to vivianite to be reached. The vivianite in the sediment contains significant amounts of manganese (∼4–8 wt.%), similar to vivianite obtained from freshwater sediments. Our results indicate that methane dynamics play a key role in providing conditions that allow for vivianite authigenesis in coastal surface sediments. We suggest that vivianite may act as an important burial sink for P in brackish coastal environments worldwide.
Fertilizer
|
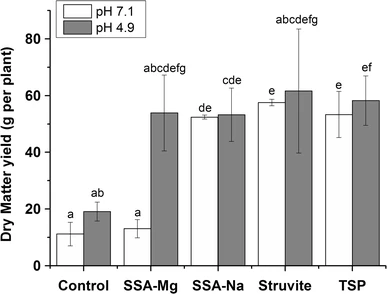
C. Vogel, C. Rivard, V. Wilken, A. Muskolus and C. Adam "Performance of secondary P-fertilizers in pot experiments analyzed by phosphorus X-ray absorption near-edge structure (XANES) spectroscopy", Ambio, 47, S62-S72 (2018). |
|
A pot experiment was carried out with maize to determine the phosphorus (P) plant-availability of different secondary P-fertilizers derived from wastewater. We analyzed the respective soils by P K-edge X-ray absorption near-edge structure (XANES) spectroscopy to determine the P chemical forms that were present and determine the transformation processes. Macro- and micro-XANES spectroscopy were used to determine the chemical state of the overall soil P and identify P compounds in P-rich spots. Mainly organic P and/or P adsorbed on organic matter or other substrates were detected in unfertilized and fertilized soils. In addition, there were indications for the formation of ammonium phosphates in some fertilized soils. However, this effect was not seen in the maize yield of all P-fertilizers. The observed reactions between phosphate from secondary P-fertilizers and co-fertilized nitrogen compounds should be further investigated. Formation of highly plant-available compounds such as ammonium phosphates could make secondary P-fertilizers more competitive to commercial phosphate rock-based fertilizers with positive effects on resources conservation.