- Home
- News
- General News
- Unveiling the structure...
Unveiling the structure of microcrystals
04-10-2007
PRESS RELEASE- Grenoble-Paris, 4 October 2007- Microcrystals are very tiny grains, so small that they form a powder. How can we determine their structure? Until today, the powerful technique of single crystal diffraction, normally used to study larger crystals, was not an appropriate solution. For the first time, researchers from the ESRF and the CNRS have used this technique to determine the structure of complex compounds in the form of microcrystals, thanks to a new equipment created at the ESRF. This breakthrough opens up new possibilities of research to chemists, physicists and biologists.
The properties of a crystal are determined by the arrangement of its atoms in space, its crystalline structure. Scientists use X-ray or neutron diffraction to study crystalline structure when the size of the crystal is more than 1000 cubic micrometres. Below this limit, the solid material is considered a powder and the technique used in that case is called “powder diffraction”. This technique is not always easy to exploit and it is not very well adapted to complex materials with a large unit cell.
|
|
|
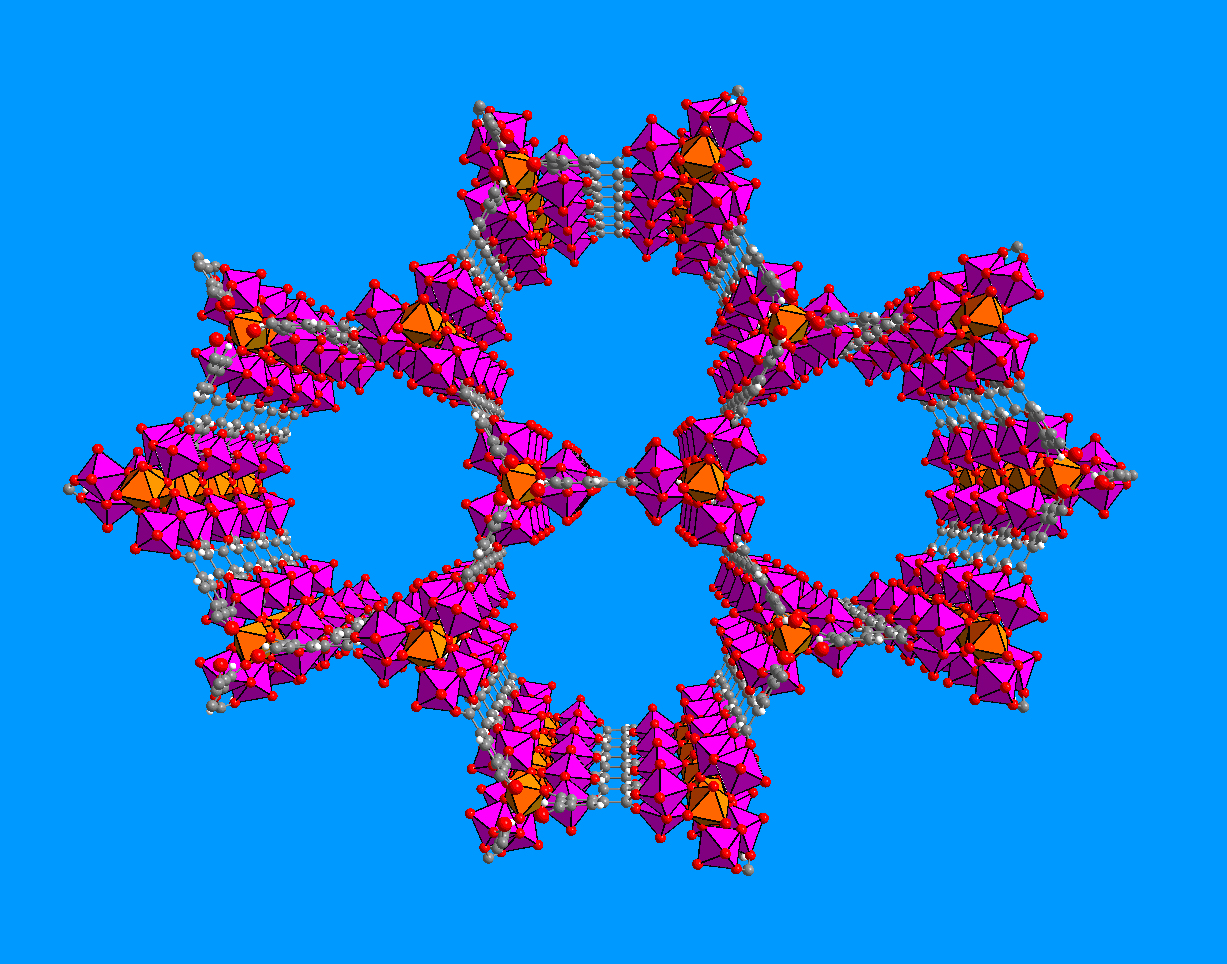
Crystalline structure of a microporous aluminium carboxylate determined at the ESRF by X-ray microdiffraction. Copyright: T. Loiseau, CNRS 2007 |
The teams from the ESRF and the Institute Lavoisier (CNRS/Université de Versailles Saint-Quentin) have used a new set-up permitting X-ray diffraction on microcrystals of microporous aluminium carboxylate. This compound is an organic-inogarnic hybrid which could be used for gas absorption or to encapsulate various organic molecules. The new ESRF set-up consists of a focussing system for the X-ray beam, coupled with a goniometer, an instrument to position the sample with maximum precision. The beam arriving onto the sample has an exceptionally small size of µm2.
This study confirms that the new set-up allows pushing back the limits of single crystal diffraction on microcrystals of complex compounds, when powder diffraction is not well adapted. “It is a revolution: what was considered a powder in the past has become a crystal today. Researchers can now bring forward samples left in their cupboards because the sizes had previously prevented their study. Now they will be able to elucidate the structures of these samples, with potentially great scientific advances on the horizon”, explains Thierry Loiseau, from the Institut Lavoisier.
A Microdiffraction Set-up for Nanoporous Metal-Organic-Framework-Type Solids. C. Volkringer, D. Popov, T. Loiseau, N. Guillou, G. Férey, M. Haouas, F. Taulelle, C. Mellot-Draznieks, M. Burghammer and C. Riekel, Nature Materials, 6 (2007) 760-4.
http://www.nature.com/nmat/journal/v6/n10/abs/nmat1991.html
Top image: A microcrystal of a microporous aluminium carboxylate (3 x 3 x 10 micrometers)