- Home
- News
- Spotlight on Science
- Marine bacteria...
Marine bacteria manage iron scarcity to produce half the world’s oxygen
30-04-2024
X-ray and neutron experiments combined with optical light spectroscopy at beamline BM07 have revealed how marine bacteria can prosper – despite having limited access to the iron needed for photosynthesis. The results show that a single iron-binding protein can bind two different forms of iron – ferric and ferrous – and reveal the mechanism by which this happens.
Photosynthetic bacteria are the main contributors to global photosynthesis. The sea is the largest ecosystem on Earth, and it harbours two photosynthetic organisms that produce approximately half of the planet’s oxygen. The cyanobacterium Prochlorococcus holds several records in the field: it is the most abundant photosynthetic organism in the oceans, and – with a diameter of half a micron – it is also the smallest photosynthetic organism on earth. Each year, it fixes approximately four gigatons of carbon, comparable to the net global primary production of the world’s agriculture industry. However, photosynthesis relies on iron, which is scarce in the ocean. The remarkable ecological success of Prochlorococcus is based on its ability to thrive in low-nutrient waters.
How do bacteria acquire iron?
To investigate how bacteria acquire iron, an international team of researchers studied crystals of the Prochlorococcus iron-binding protein, FutA, using several complementary structural biology techniques. First, neutron crystallography was used to locate hydrogen atoms around FutA’s iron-binding site, which allowed determination of the charges of amino acid side chains and the charge state of iron.
Optical spectroscopy at BM07
Next, the conversion of iron between oxidation states in the protein was investigated using optical spectroscopy measurements carried out at ESRF beamline BM07, with the help of the in crystallo Optical Spectroscopy (icOS) laboratory. Researchers irradiated the protein crystals with X-rays and observed how the protein with bound iron loses colour, converting from rust-red, oxidised (ferric) iron to colourless, reduced (ferrous) iron (Figures 1a and b).
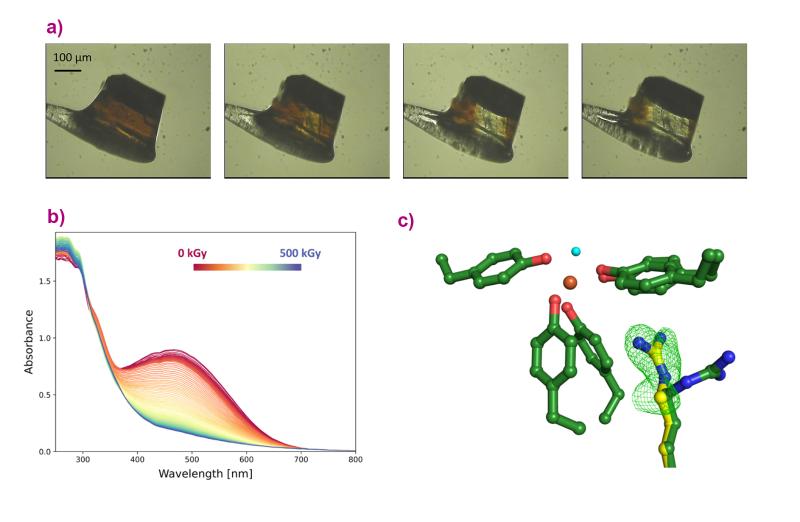
Fig. 1: a) A crystal of FutA iron-binding protein loses colour when it is exposed to X-rays. b) This effect was quantified at the ESRF beamline BM07, by performing spectroscopy measurements on the FutA crystal during X-ray exposure, plotted from 0 kGy (red) to 500 kGy (blue). The spectra flatten with increasing X-ray dose. c) The dose required to switch between the two forms of iron was then used in an X-ray pump-probe experiment at the SACLA XFEL facility to see how the protein responded to this change. The data reveal an amino acid side chain moving towards the iron, as indicated by the green mesh (c). The structure shown with green carbon atoms was observed before irradiation with X-rays; the one with yellow carbons after. Oxygen is shown in red; nitrogen in blue; iron in orange, and the water molecule in light blue.
The protein crystals were then exposed to specific X-ray doses, and their crystal structures were measured using serial synchrotron crystallography at Diamond Light Source in the UK and at the SACLA X-ray free-electron laser (XFEL) facility in Japan. The results show changes in the structure of the protein bound to iron before and after X-ray exposure, revealing that the protein makes a ’structural switch’ in order to bind both forms of iron (Figure 1c).
One protein, two functions
This research demonstrates that, surprisingly, the single FutA protein can perform two essential functions. Cyanobacteria typically have two types of proteins for these functions, namely the uptake of oxidised iron from the environment and the protection of the bacterial photosystems in reduced form. The finding that Prochlorococcus has a single protein for two functions may be an important factor for its ecological success and relate to the limited number of genes in the greatly reduced genome of the tiny bacterium, as well as revealing crucial insight into the biology of marine bacteria.
Principal publication and authors
A redox switch allows binding of Fe(II) and Fe(III) ions in the cyanobacterial iron-binding protein FutA from Prochlorococcus, R. Bolton (a,b), M.M. Machelett (a,c), J. Stubbs (a,b), D. Axford (b), N. Caramello (d,e), L. Catapano (f,g), M. Malý (a), M.J. Rodrigues (a,b,h), C. Cordery (a,b), G.J. Tizzard (i), F. MacMillan (j), S. Engilberge (d,k), D. von Stetten (l), T. Tosha (m), H. Sugimoto (m), J.A.R. Worrall (n), J.S. Webb (a,o), M. Zubkov (c,p), S. Coles (i), E. Mathieu (k), R.A. Steiner (f,q), G. Murshudov (g), T.E. Schrader (r), A.M. Orville (b,s), Antoine Royant (d,k), G. Evans (b,t), M.A Hough (b,n,s), R.L. Owen (b), I. Tews (a), Proc. Natl. Acad. Sci. USA, 121(12), e2308478121 (2024); https://doi.org/10.1073/pnas.2308478121
(a) Biological Sciences, Institute for Life Sciences, University of Southampton, Southampton (UK)
(b) Diamond Light Source, Harwell Science and Innovation Campus, Didcot (UK)
(c) National Oceanography Centre, Southampton (UK)
(d) ESRF
(e) Hamburg Centre for Ultrafast Imaging, Hamburg Advanced Research Centre for Bioorganic Chemistry, Universität Hamburg, Hamburg (Germany)
(f) Randall Centre of Cell and Molecular Biophysics, King's College London, London (UK)
(g) Medical Research Council Laboratory of Molecular Biology, Cambridge (UK)
(h) Laboratory of Biomolecular Research, Paul Scherrer Institute, Villigen (Switzerland)
(i) School of Chemistry, University of Southampton, Southampton (UK)
(j) School of Chemistry, University of East Anglia, Norwich (UK)
(k) Univ. Grenoble Alpes, CNRS, CEA, Institut de Biologie Structurale, Grenoble (France)
(l) European Molecular Biology Laboratory, Hamburg Unit, Hamburg (Germany)
(m) Synchrotron Radiation Life Science Instrumentation Team, RIKEN SPring-8 Center, Sayo, Hyogo (Japan)
(n) School of Life Sciences, University of Essex, Colchester (UK)
(o) National Biofilms Innovation Centre (NBIC), University of Southampton, Southampton (UK)
(p) Scottish Association for Marine Science, Oban (UK)
(q) Department of Biomedical Sciences, University of Padova, Padova (Italy)
(r) Forschungszentrum Jülich GmbH, Jülich Centre for Neutron Science, Garching (Germany)
(s) Research Complex at Harwell, Harwell Science and Innovation Campus, Didcot (UK)
(t) Rosalind Franklin Institute, Harwell Science and Innovation Campus, Didcot (UK)
| About the beamlines |
| BM07 |
| The CRG beamline BM07-FIP2 (French beamline for Investigation of Proteins) is dedicated to crystallography of biological macromolecules. Its optics can deliver a top-hat beam on a fixed sample position in a large energy range (6-20 keV). BM07 offers high-throughput screening of cryocooled crystals, anomalous diffraction experiments, on-line UV-Vis absorption microspectrophotometry and humidity-controlled experiments. |
| icOS Laboratory |
| The icOS Lab is dedicated to various types of optical spectroscopy experiments on macromolecular crystals, performed either in an offline facility (UV-Vis absorption, pump-probe microsecond UV-Vis absorption, fluorescence emission, Raman), or with microspectrophotometers directly mounted on beamlines BM07 (UV-Vis absorption, fluorescence emission) or ID30B (Raman). The lab is being run within the framework of a close collaboration between the ESRF and the Institut de Biologie Structurale (IBS), and is one of the platforms of the Partnership for Structural Biology (PSB). |



