- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Soft condensed matter
- Fast nucleation and growth of nanocrystals of a porous coordination polymer
Fast nucleation and growth of nanocrystals of a porous coordination polymer
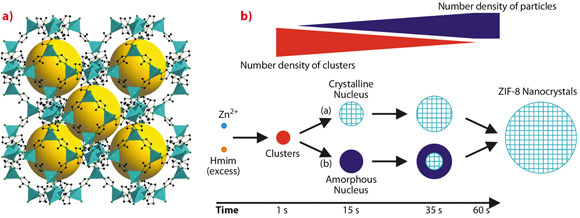
Metal-organic frameworks (MOFs) constitute a novel class of crystalline porous materials with many potential applications in gas storage, separation, catalysis and medical diagnostics. The three-dimensional frameworks of these hybrid materials are assembled from inorganic units and bridging polytopic organic ligands. A widely investigated MOF is the microporous zeolitic imidazolate framework 8 (ZIF-8). ZIF-8 consists of zinc cations and bridging 2-methylimidazolate anions (Figure 54a). ZIF-8 exhibits outstanding thermal and chemical stability: it can be heated up to 400°C in air and resists boiling solvents like benzene, water and dilute aqueous NaOH for periods of many hours. The small pores of ZIF-8 enable the separation of hydrogen from larger gas molecules.
At present, the mechanisms of MOF crystallisation are only poorly understood. This significantly limits the design of new MOF compounds as well as the control of the size and morphology of MOF particles. ZIF-8 nanocrystals with a narrow size distribution can be prepared rapidly by simply combining methanol solutions of zinc salt and organic ligand at room temperature. Monitoring the process in situ by time-resolved static light scattering (SLS) revealed nanoparticles with a size of approximately 45 nm at 130 seconds after the start of the reaction [1]. Unfortunately, the very early events occurring before 130 seconds could not be followed by SLS. It is this initial phase, which now became accessible by combined in situ SAXS/WAXS experiments at beamline ID02 with a time resolution of 1 second. A stopped-flow device available at ID02 was used to rapidly mix the two component solutions and thus defined the onset of the fast reaction as precisely as possible. The diagram in Figure 54b presents the various species detected by SAXS/WAXS and possible alternative crystallisation pathways.
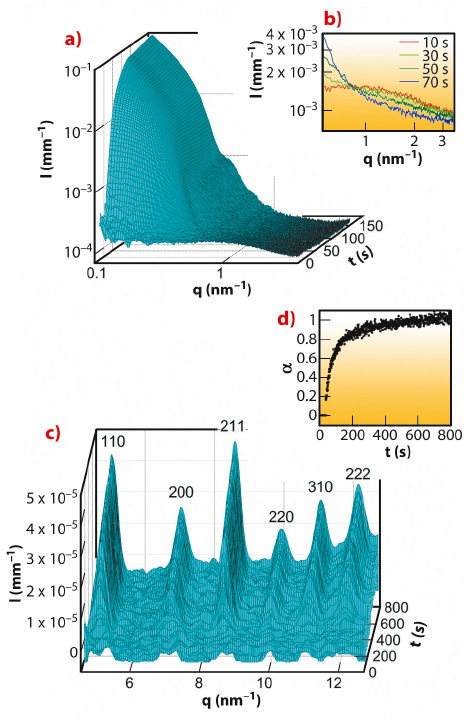
The SAXS patterns (Figure 55a) reveal that small nanoparticles with a diameter of approximately 2 nm (denoted clusters) were present already after 1 second (first measurement), while larger ZIF-8 particles started to form after 15 seconds and had grown to a size of approximately 25 nm at 800 seconds. The SAXS intensities corresponding to the clusters and particles are inversely correlated (Figure 55b) suggesting that the clusters were involved in the particle formation processes. Quantitative SAXS data evaluation indicated that the clusters took part also in particle nucleation. The particles grew by a monomer addition mechanism with the clusters and/or smaller units acting as the monomers but not by coalescence. Bragg peaks are first seen in the WAXS patterns after 35 seconds (Figure 55c) that is 7 seconds after the emergence of the first particles. This may be taken as an indication that the very first ZIF-8 particles were amorphous,and that crystalline order developed later by internal structural rearrangement (pathway b in Figure 54b). A plot of the extent of crystallisation as a function of time (Figure 55d) shows that the fast crystallisation process slowed down after approximately 300 seconds and was followed by a slower process, which most likely is Ostwald ripening.
 |
|
Fig. 55: a) SAXS patterns for the first 150 seconds. b) High-q region of selected SAXS patterns originating from the small clusters of approximately 2 nm; the time at which each pattern was measured is indicated. c) WAXS patterns between 1 and 800 seconds; all Bragg reflection belong to the cubic body-centred lattice of ZIF-8. d) Extent of crystallisation (α) as a function of time (t). |
The SAXS/WAXS experiments enabled direct observation of MOF nucleation and early growth in homogeneous solution for the first time. The ZIF-8 nanocrystal formation observed does not follow classical nucleation theory and exhibits similarities with the crystallisation processes of some high-silica zeolites from clear solutions. A number of questions remain to be answered concerning, for example, the chemical composition and structure of the clusters and the detailed mechanism by which the clusters contribute to nucleation and/or growth of the ZIF-8 particles.
Principal publication and authors
J. Cravillon (a), C.A. Schröder (a), R. Nayuk (b), J. Gummel (c), K. Huber (b) and M. Wiebcke (a), Angew. Chem., Int. Ed. 50, 8067-8071 (2011).
(a) Institut für Anorganische Chemie, Leibniz Universität Hannover (Germany)
(b) Department Chemie, Universität Paderborn (Germany)
(c) ESRF
References
[1] J. Cravillon, R. Nayuk, S. Springer, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater. 23, 2130-2141 (2011).